Human mesenchymal stem cell-based extracellular matrix-derived composite collagen extracting solution hydrogel and preparation method thereof
A technology of mesenchymal stem cells and extracellular matrix, applied in the fields of pharmaceutical formula, medical science, prosthesis, etc., can solve the problems of poor mechanical strength, achieve the effect of improving mechanical strength, good biocompatibility, and preventing cell loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0036] According to a typical embodiment of the present invention, there is provided a method for preparing a composite collagen extract hydrogel based on human mesenchymal stem cell extracellular matrix, said method comprising:
[0037] Dissolving the human mesenchymal stem cell extracellular matrix-derived composite collagen extract in the buffer solution to obtain a protein solution with a mass concentration of 0-100 mg / mL;
[0038] Adding the protein solution to natural molecules and synthetic molecules and mixing to obtain a mixed solution; the final concentration of the synthetic molecules is 0-100 mg / mL; the final concentration of the natural molecules is 0-100 mg / mL;
[0039] Adding the mixed solution to a cross-linking activator and mixing to carry out a cross-linking reaction to obtain a cross-linking reaction solution; the final concentration of the cross-linking activator used is 1-30 mg / mL;
[0040] The cross-linking reaction solution is purified to obtain a compo...
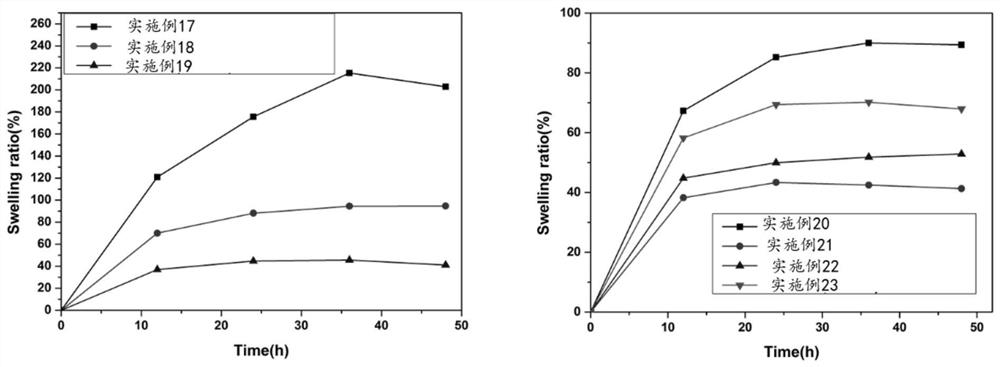
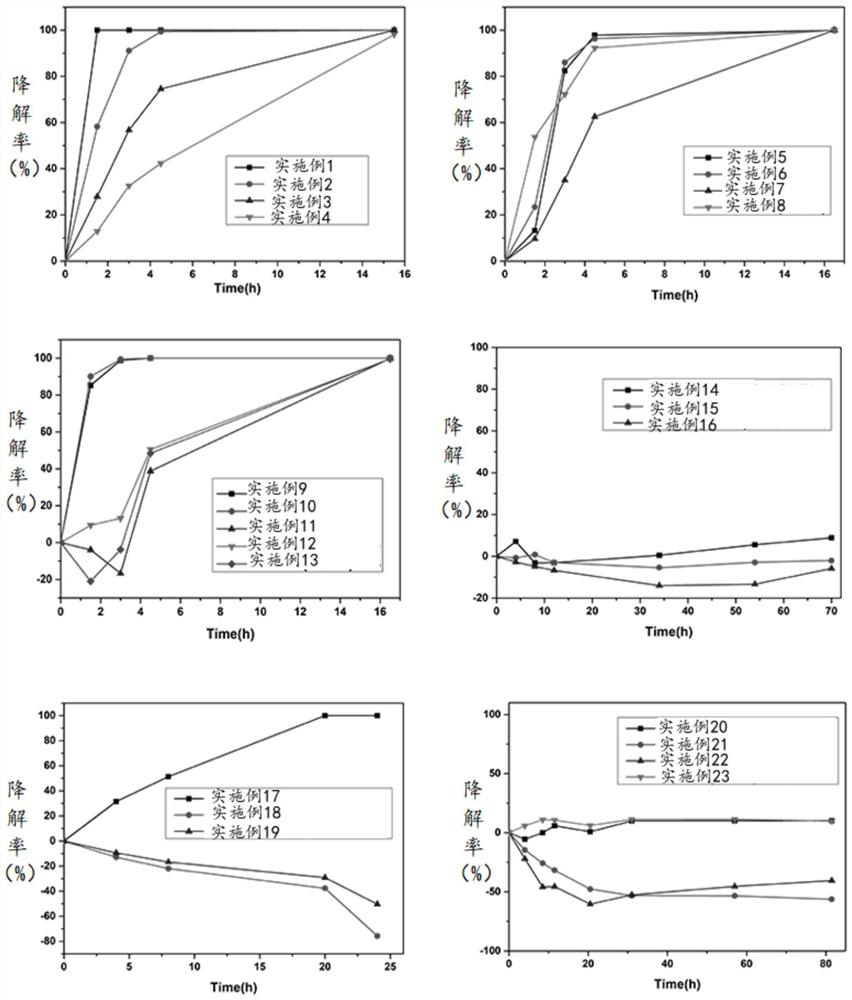
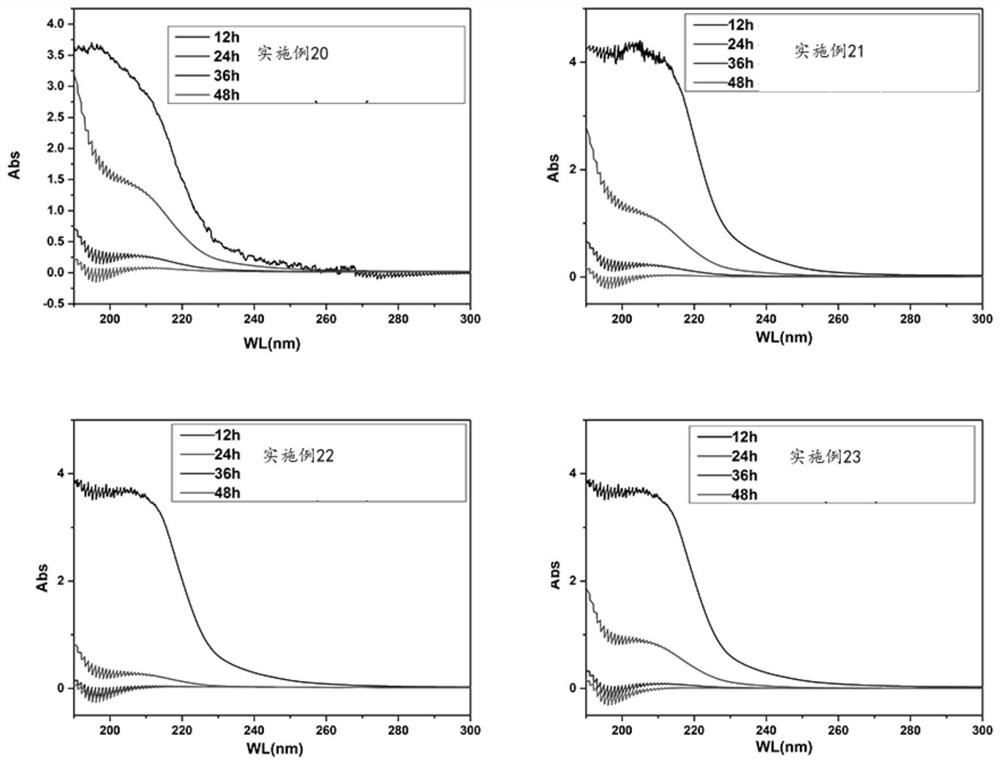
Embodiment 1
[0076] Step S1, prepare 100mM MES buffer solution, and adjust the pH to 6-7 with 1M NaOH.
[0077] Step S2, adding human mesenchymal stem cell extracellular matrix-derived composite collagen extract, making the mass concentration of human mesenchymal stem cell extracellular matrix-derived composite collagen extract in the mixed solution 50 mg / mL, and stirring evenly;
[0078] Step S3, adding EDC, making the mass concentration of the activator in the mixed solution to be 1 mg / mL, stirring evenly, and reacting at room temperature for 4-6 hours to obtain the hydrogel ECM 50-EDC 1 .
Embodiment 2
[0080] Step S1, prepare 100mM MES buffer solution, and adjust the pH to 6-7 with 1M NaOH.
[0081] Step S2, adding human mesenchymal stem cell extracellular matrix-derived composite collagen extract, making the mass concentration of human mesenchymal stem cell extracellular matrix-derived composite collagen extract in the mixed solution 50 mg / mL, and stirring evenly;
[0082] Step S3, adding EDC so that the mass concentration of the activator in the mixed solution is 3 mg / mL, stirring evenly, and reacting at room temperature for 4-6 hours to obtain the hydrogel ECM 50-EDC 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com