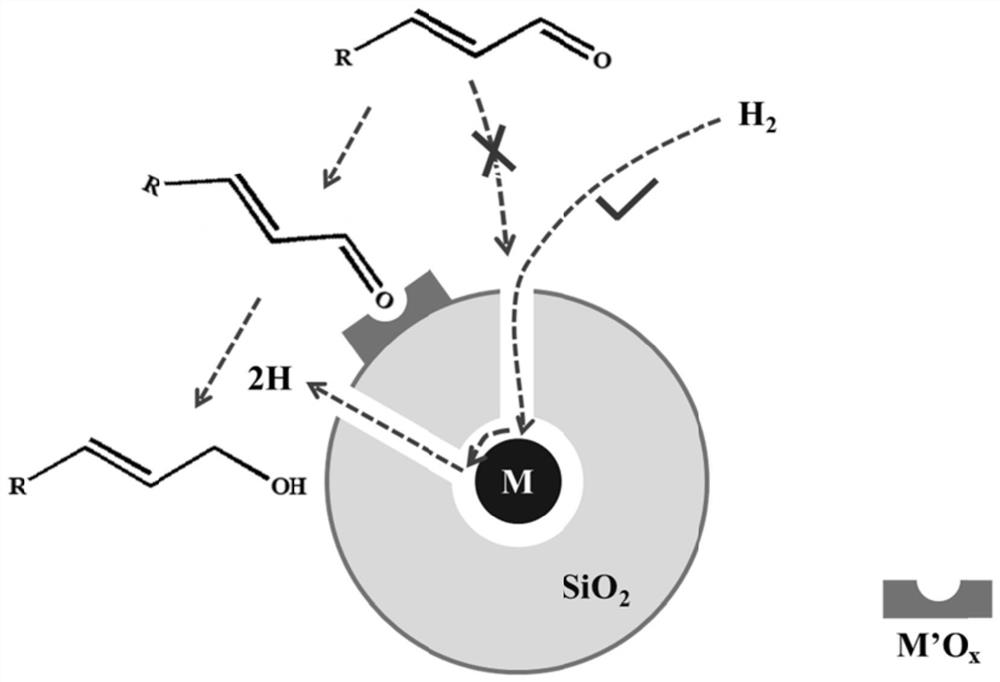
Alpha,beta-unsaturated aldehyde selective hydro-conversion catalyst as well as preparation method and application thereof
A technology for aldehyde selectivity and hydrogenation conversion, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. Solve problems such as leaching and catalyst deactivation, and achieve the effects of adjustable pore size, easy synthesis, improved reaction selectivity, and high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A preparation method of α, β-unsaturated aldehyde selective hydrogenation conversion catalyst, comprising the following steps:
[0030] (1) Take 0.08g CuO and disperse it in 100mL of absolute ethanol, slowly add 1.50g of polyvinylpyrrolidone;
[0031] (2) After the above-mentioned mixture was stirred at room temperature for 12 hours and mixed evenly, it was added to a mixed solution containing 50mL of deionized water, 50mL of absolute ethanol, and 4mL of ammonia water (28wt%), and 1.5g of cetyl tris Methyl ammonium bromide and 3.0g tetraethyl orthosilicate, stirred at room temperature for 12h, mixed evenly, transferred to a hydrothermal reaction kettle, reacted at 120°C for 15h, cooled naturally, centrifuged and washed the reaction liquid, Dry at 80°C for 15 hours to obtain CuO@SiO 2 ;
[0032] (3) CuO@SiO obtained in step (2) 2 Disperse in 30mL aqueous solution containing 0.20g cerium nitrate hexahydrate, stir at room temperature for 15h, dry at 80°C for 15h, and ro...
Embodiment 2
[0036] A preparation method of α, β-unsaturated aldehyde selective hydrogenation conversion catalyst, comprising the following steps:
[0037] (1) Take 0.06g PtO 2 Disperse in 90mL of absolute ethanol, slowly add 1.30g of polyvinylpyrrolidone;
[0038] (2) After the above-mentioned mixed material was stirred at room temperature for 10 h and mixed evenly, it was added to a mixed solution containing 45 mL of deionized water, 45 mL of absolute ethanol, and 3.7 mL of ammonia water (28 wt%), and 1.2 g of hexadecyl was added during the stirring process. Trimethylammonium bromide and 2.8g tetraethyl orthosilicate, stirred at room temperature for 10h, mixed evenly, transferred to a hydrothermal reaction kettle, reacted at 180°C for 10h, cooled naturally, centrifuged and washed the reaction solution , dried at 120°C for 10 h to obtain PtO 2 @SiO 2 ;
[0039] (3) the PtO obtained in step (2) 2 @SiO 2 Disperse in 20mL aqueous solution containing 0.30g indium nitrate, stir at room t...
Embodiment 3
[0043] A preparation method of α, β-unsaturated aldehyde selective hydrogenation conversion catalyst, comprising the following steps:
[0044] (1) Take 0.15g NiO and disperse it in 110mL absolute ethanol, slowly add 1.70g polyvinylpyrrolidone;
[0045] (2) After the above mixed material was stirred at room temperature for 15 hours and mixed evenly, it was added to a mixed solution containing 55mL of deionized water, 55mL of absolute ethanol, and 4.2mL of ammonia water (28wt%), and 1.8g of hexadecyl was added during the stirring process. Trimethylammonium bromide and 3.2g ethyl orthosilicate, stirred at room temperature for 15h, mixed evenly, transferred to a hydrothermal reaction kettle, reacted at 140°C for 14h, cooled naturally, centrifuged and washed the reaction solution , dried at 90°C for 14h to obtain NiO@SiO 2 ;
[0046] (3) NiO@SiO obtained in step (2) 2 Disperse in 55mL aqueous solution containing 0.35g ammonium metavanadate, stir at room temperature for 8h, dry a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com