Preparation method of 1, 3, 5-trimethoxybenzene
A technology of trimethoxybenzene and methanol, which is applied in ether preparation, ester reaction preparation of ether, organic chemistry, etc., can solve the problems of cumbersome operation, high preparation cost, and low purity, and achieve high yield and purity, and stable product quality , good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of crude 1,3,5-trimethoxybenzene
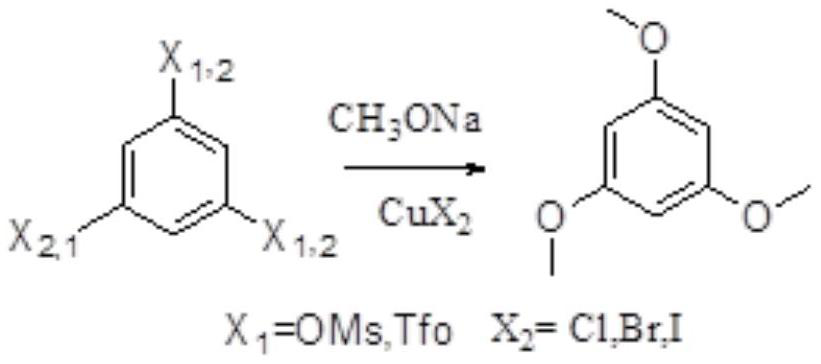
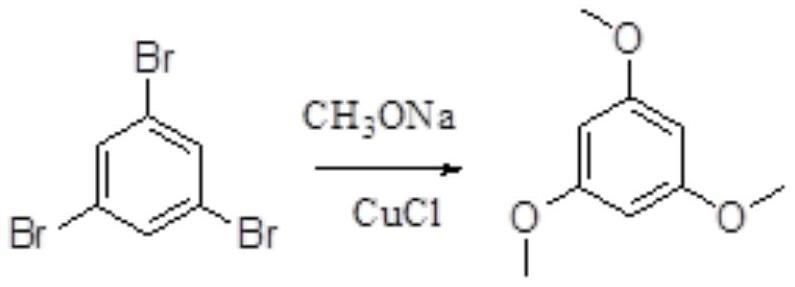
[0044] Add 150 mL of N,N-dimethylamide to the compound 1,3,5-tribromobenzene (100.0 g, 0.32 mol), add 300 mL of methanol under stirring conditions, and add sodium methoxide (102.9 g, 1.91 mol), After the addition, the temperature was raised to 60-70°C, cuprous chloride (1.58g, 0.016mol) was added, the temperature was raised to 90-95°C, after the reaction was completed, it was cooled to room temperature, the insoluble solid was filtered out, and the filtrate was added to sulfuric acid (0.76 mL, 0.95mol / L), stirring and crystallizing overnight, and then filtering out the solid, and vacuum-drying the obtained solid at 45°C for 8 hours to obtain 44.3 g of off-white solid.
[0045] The reaction formula is as follows:
[0046]
[0047] Purification of 1,3,5-trimethoxybenzene
[0048] Add 200mL of water to the crude 1,3,5-trimethoxybenzene (20g, 0.012mol) obtained in the reaction, stir for 2 hours and filter out the solid, add ...
Embodiment 2
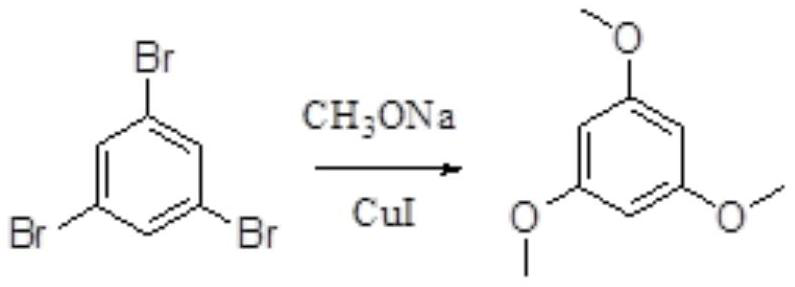
[0067] Catalyst selects cuprous iodide (the mol ratio of catalyst and reaction substrate is the same as embodiment 1), and the remainder is the same as embodiment 1.
[0068]
[0069] Obtained off-white solid 16.2 g of off-white solid with a purity of 98.5%.
Embodiment 3
[0071] The reaction substrate is selected from 1,3,5-triiodobenzene, the catalyst is selected from cuprous iodide (the molar ratio of the catalyst to the reaction substrate is the same as in Example 1), and the remainder is the same as in Example 1.
[0072]
[0073] Obtained off-white solid 16.1 g of off-white solid with a purity of 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com