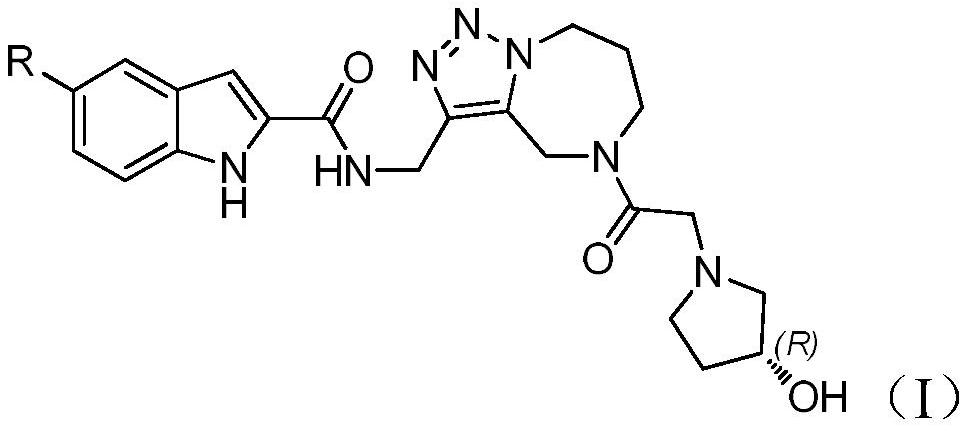
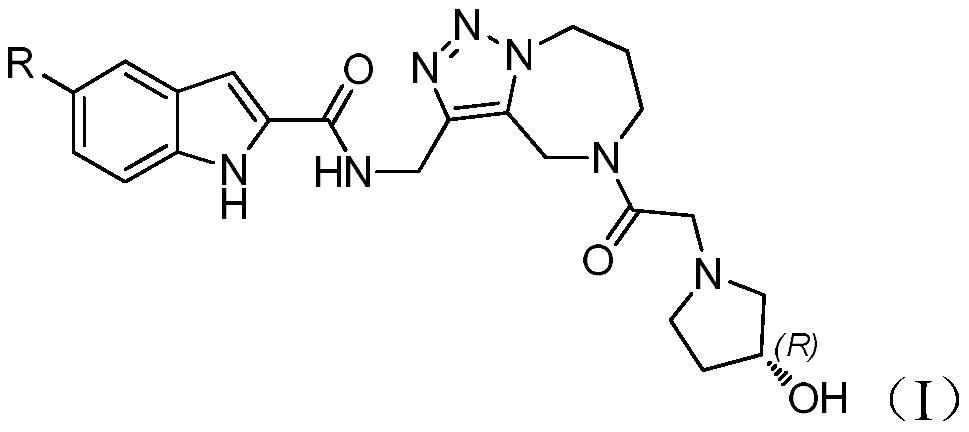
Complex of azepine-fused triazole ring and indole carboxylic acid and salt, preparation method and medical application thereof
A technology for compounds and mixtures, applied in the field of their preparation, complexes of heterozotriazole rings and indole carboxylic acids and their salts, can solve problems such as no effective medicines yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 3-[(5-chloro-1H-indole-2-carboxamido)methyl]-7,8-dihydro-4H-[1,2,3]triazole[1,5-a ][1,4]diazepine-5(6H)-tert-butyl carboxylate
[0039]
[0040]Dissolve 5-chloroindole-2-carboxylic acid (200mg, 1.02mmol) in DMF (10mL), add EDCI (235mg, 1.23mmol), HOBT (165.6mg, 1.23mmol), DIPEA (0.54mL, 3.08mmol), under nitrogen protection, stirred at 0°C for 30min, added 3-(aminomethyl-7,8-dihydro-4H-[1,2,3]triazole[1,5-a][1,4 ]diazepine-5(6H)-tert-butyl carboxylate (272.3mg, 1.02mmol), then stirred overnight at room temperature, poured the reaction solution into ice water, extracted with ethyl acetate (15mL×3), and washed with saturated salt The organic phase was washed with water (15mL×3), dried over anhydrous magnesium sulfate for 2-3h, and subjected to silica gel column chromatography (CH 2 Cl 2 / CH 3 OH 100 / 3, V / V) to give a white solid (400mg, 88%).
Embodiment 2
[0041] Example 2 N-[(5,6,7,8-tetrahydro-4H-[1,2,3]triazol[1,5-a][1,4]diazepine-3-yl) Methyl]-5-chloro--1H-indole-2-carboxamide
[0042]
[0043] 3-[(5-chloro-1H-indole-2-carboxamido)methyl]-7,8-dihydro-4H-[1,2,3]triazole[1,5-a][ 1,4] Diazepine-5(6H)-tert-butyl carboxylate (400 mg, 0.90 mmol) was dissolved in 60 mL of dichloromethane, and 4 mL of trifluoroacetic acid was added dropwise in an ice-water bath. After 30 min, the ice-water bath was removed, and the Stir for 4 h, stop the reaction, evaporate the solvent to dryness under reduced pressure, dissolve the residue with 30 mL of ethyl acetate, wash the organic phase with saturated sodium bicarbonate solution and saturated brine (15 mL×3), and dry over anhydrous magnesium sulfate for 2-3 h. Concentrate, dry the sample, and take it directly to the next step without further purification.
Embodiment 3
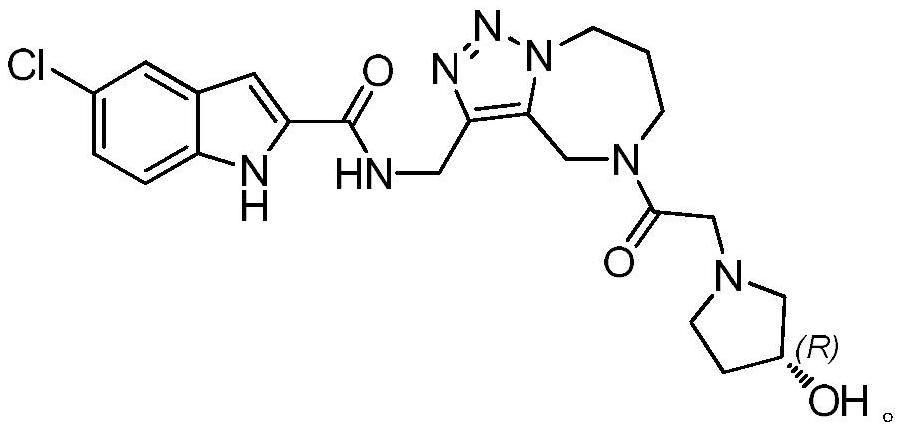
[0044] Example 3 (R)-N-({5-[2-(3-hydroxypyrrolidin-1-yl)acetyl]-5,6,7,8-tetrahydro-4H-[1,2,3 ]triazol[1,5-a][1,4]diazepine-3-yl}methyl)-5-chloro-1H-indole-2-carboxamide
[0045]
[0046] Dissolve (R)-2-(3-hydroxypyrrolidin-1-yl)acetic acid (145mg, 1mmol) in DMF (8mL), add EDCI (230mg, 1.2mmol) while stirring, HOBT (162mg, 1.2mmol ), DIPEA (0.52mL, 3mmol), under nitrogen protection, stirred for 30min at 0°C, added N-[(5,6,7,8-tetrahydro-4H-[1,2,3]triazole[1,5 -a][1,4]diazepine-3-yl)methyl]-5-chloro-1H-indole-2-carboxamide (150.5mg, 0.50mmol), then stirred at room temperature overnight, and the reaction solution Pour into ice water, extract with ethyl acetate (15mL×3), wash the organic phase with saturated brine (15mL×3), dry over anhydrous magnesium sulfate for 2-3h, perform silica gel column chromatography (CH 2 Cl 2 / CH 3 OH100 / 3, V / V) to obtain a white solid (117.8 mg, 50%).
[0047] HPLC analysis: 98.5%.
[0048] ESI-MS m / z:472.1(M+H)+.
[0049] 1 H-NMR(400MHz,d6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com