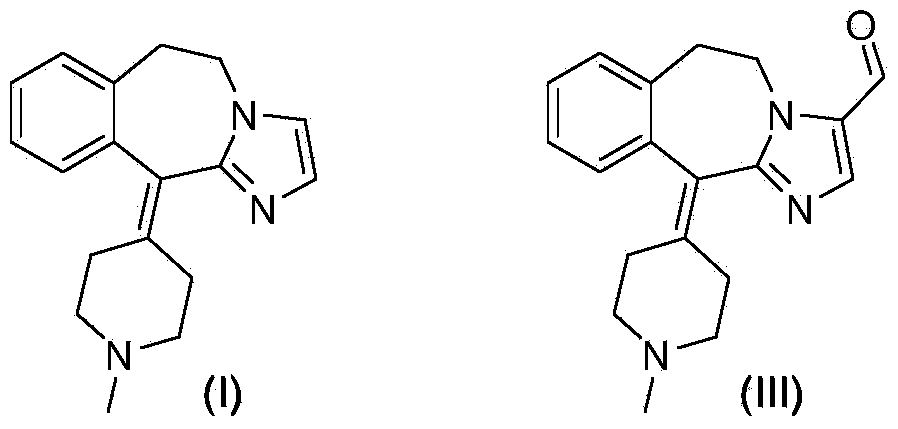
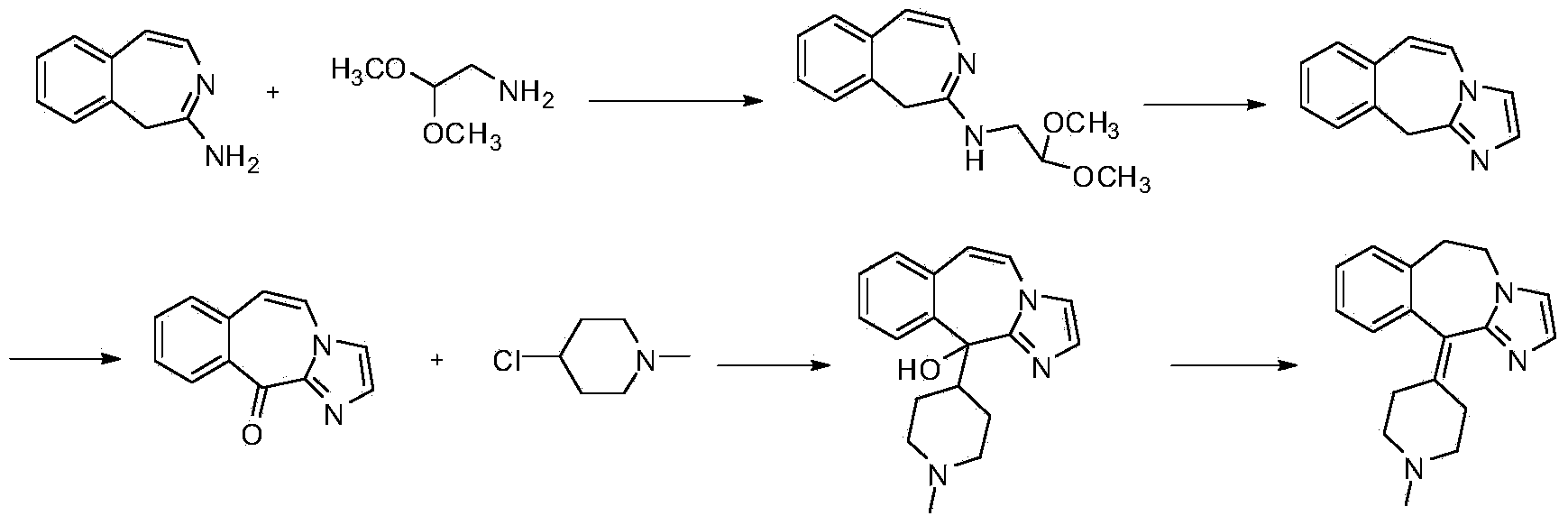
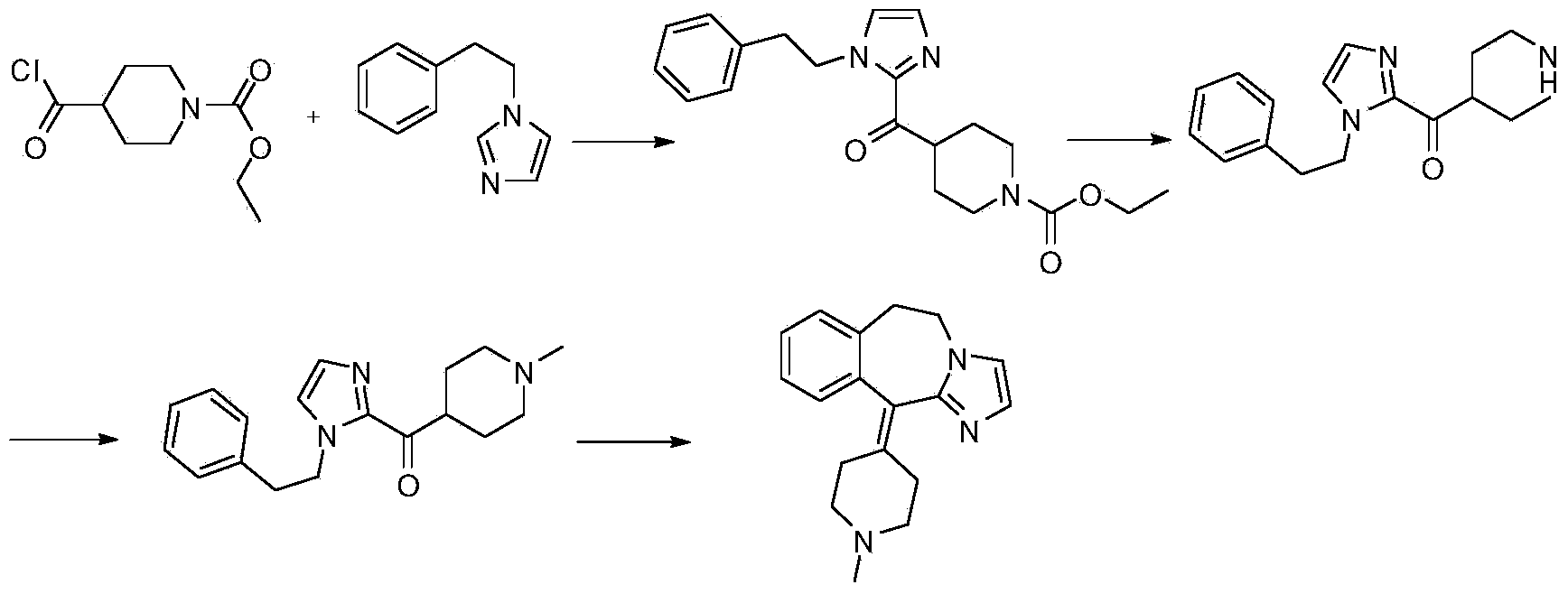
Preparation method of alcaftadine intermediate
A reaction system, methyl technology, applied in the direction of organic chemistry, etc., can solve the problems of reduced safety requirements, long route, low overall yield, etc., and achieve the effect of increased reaction yield, shortened reaction route, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Add 86g of 1-(2-phenylethyl)-1H-imidazole, 500ml of acetonitrile, and 120g of triethylamine into a 1000ml three-neck flask, cool down to 0-5°C in an ice-water bath, and slowly add N-methyl-4 - 108g of piperidinecarbonyl chloride, after the dropwise addition, warm up to room temperature (25-30°C), keep warm until the reaction is complete, add 1L of 20% sodium hydroxide and stir for 30min, extract with dichloromethane, dry over anhydrous sodium sulfate, filter, The solvent was recovered from the filtrate to obtain 134.1 g of the product [1-(2-phenylethyl)-1H-imidazol-2-yl](1-methyl-4-piperidinyl)methanone, with a yield of 90.3%.
[0034] (2) Add 87g of [1-(2-phenylethyl)-1H-imidazol-2-yl](1-methyl-4-piperidinyl)methanone into a 500ml three-necked flask, and then add 350ml of polyphosphoric acid , heated to 110°C, kept warm for 12 hours, after the reaction was completed, cooled to room temperature, poured into 500ml of ice water, 40% sodium hydroxide solution to pH=10-...
Embodiment 2
[0036] (1) Add 86g of 1-(2-phenylethyl)-1H-imidazole, 200ml of pyridine, and 60g of triethylamine into a 500ml three-neck flask, cool down to 0-5°C in an ice-water bath, and slowly add N-methyl-4 - 161g of piperidinecarbonyl chloride, after the dropwise addition, heat up to 65°C, keep warm until the reaction is complete, spin dry the solvent, add 100ml of 20% sodium hydroxide and stir for 30 minutes, extract with dichloromethane, dry over anhydrous sodium sulfate, filter , The solvent was recovered from the filtrate to obtain 139.7 g of the product [1-(2-phenylethyl)-1H-imidazol-2-yl](1-methyl-4-piperidinyl)methanone, with a yield of 94.1%.
[0037] (2) Add 90g of [1-(2-phenylethyl)-1H-imidazol-2-yl](1-methyl-4-piperidinyl)methanone into a 1000ml three-necked flask, and then add 48% hydrogen Bromic acid 500ml, heated to reflux, heated to 120°C and kept warm until the reaction was complete, the reaction time was 5-17 hours, evaporated the solvent, added 500ml isopropanol to ref...
Embodiment 3
[0039] (1) Add 86g of 1-(2-phenylethyl)-1H-imidazole, 200ml of toluene, and 160g of pyridine into a 500ml three-neck flask, cool down to 0-5°C in an ice-water bath, and slowly add N-methyl-4-piperide dropwise 161g of picoyl chloride, after the dropwise addition, heat up to 95°C, keep warm until the reaction is complete, spin to dry the solvent, add 100ml of 20% sodium hydroxide and stir for 30 minutes, extract with dichloromethane, dry over anhydrous sodium sulfate, filter, and the filtrate The solvent was recovered to obtain 135.7 g of the product [1-(2-phenylethyl)-1H-imidazol-2-yl](1-methyl-4-piperidinyl)methanone, with a yield of 91.4%.
[0040] (2) Add 56g of [1-(2-phenylethyl)-1H-imidazol-2-yl](1-methyl-4-piperidinyl)methanone into a 500ml three-necked flask, and then add trifluoroform 350ml of sulfonic acid, heat to 100°C, keep warm until the reaction is complete, the reaction time is 3-7 hours, cool to room temperature, pour 500ml of ice water, 40% sodium hydroxide sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com