Synthesis method for synthesizing 4-ethynyl-tetrahydropyran from 2, 2-dichloroethyl ether
A synthetic method, tetrahydropyran technology, applied in chemical instruments and methods, silicon organic compounds, compounds of group 4/14 elements of the periodic table, etc., can solve problems such as complicated operation, high cost, unsuitable for industrial production, etc. , to achieve the effect of simple operation, low cost and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
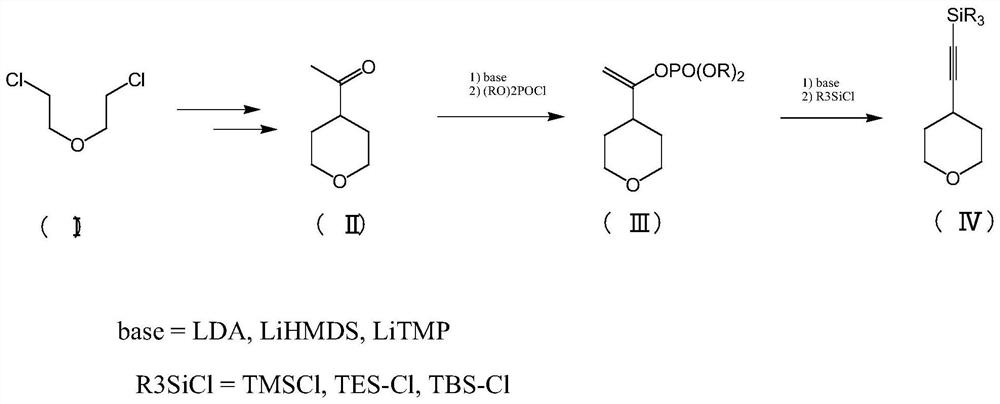
[0037] see figure 1 , this example provides a synthesis method of 4-ethynyl-tetrahydropyran starting from 2,2'-dichloroethyl ether, the specific steps are:
[0038] Under nitrogen protection, compound I (100g, 699.3mmol) was dissolved in DMF (400mL), potassium carbonate (193g, 1398.5mmol) and potassium iodide (58g, 349.6mmol) were added, the temperature was raised to 75-80°C, and acetoacetic acid was added dropwise Ethyl ester (109.2g, 839.1mmol), control the temperature at 80-85°C, keep the temperature for 15-20h, filter after the reaction is completed, add 400g water and 300g ethyl acetate to the filtrate, take the organic phase, and use 300g ethyl acetate for the water phase Extract twice, combine the organic phases, wash twice with 300mL water, wash once with 100mL saturated brine, and remove the solvent to obtain 146g of the product; 730g of 30% sulfuric acid is added dropwise to the product of the previous step, and react at 100-110°C for 15-18h. 300g of dichloromethane...
Embodiment 2
[0043] see figure 1 , this example provides a synthesis method of 4-ethynyl-tetrahydropyran starting from 2,2'-dichloroethyl ether, the specific steps are:
[0044] Under nitrogen protection, compound I (100g, 699.3mmol) was dissolved in DMF (400mL), potassium carbonate (193g, 1398.5mmol) and potassium iodide (58g, 349.6mmol) were added, the temperature was raised to 75-80°C, and acetoacetic acid was added dropwise Ethyl ester (109.2g, 839.1mmol), control the temperature at 80-85°C, keep the temperature for 15-20h, filter after the reaction is completed, add 400g water and 300g ethyl acetate to the filtrate, take the organic phase, and use 300g ethyl acetate for the water phase Extract twice, combine the organic phases, wash twice with 300mL water, wash once with 100mL saturated brine, and remove the solvent to obtain 146g of the product; 730g of 30% sulfuric acid is added dropwise to the product of the previous step, and react at 100-110°C for 15-18h. 300g of dichloromethane...
Embodiment 3
[0048] see figure 1 , this example provides a synthesis method of 4-ethynyl-tetrahydropyran starting from 2,2'-dichloroethyl ether, the specific steps are:
[0049] Under nitrogen protection, compound I (100g, 699.3mmol) was dissolved in DMF (400mL), potassium carbonate (193g, 1398.5mmol) and potassium iodide (58g, 349.6mmol) were added, the temperature was raised to 75-80°C, and acetoacetic acid was added dropwise Ethyl ester (109.2g, 839.1mmol), control the temperature at 80-85°C, keep the temperature for 15-20h, filter after the reaction is completed, add 400g water and 300g ethyl acetate to the filtrate, take the organic phase, and use 300g ethyl acetate for the water phase Extract twice, combine the organic phases, wash twice with 300mL water, wash once with 100mL saturated brine, and remove the solvent to obtain 146g of the product; 730g of 30% sulfuric acid is added dropwise to the product of the previous step, and react at 100-110°C for 15-18h. 300g of dichloromethane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com