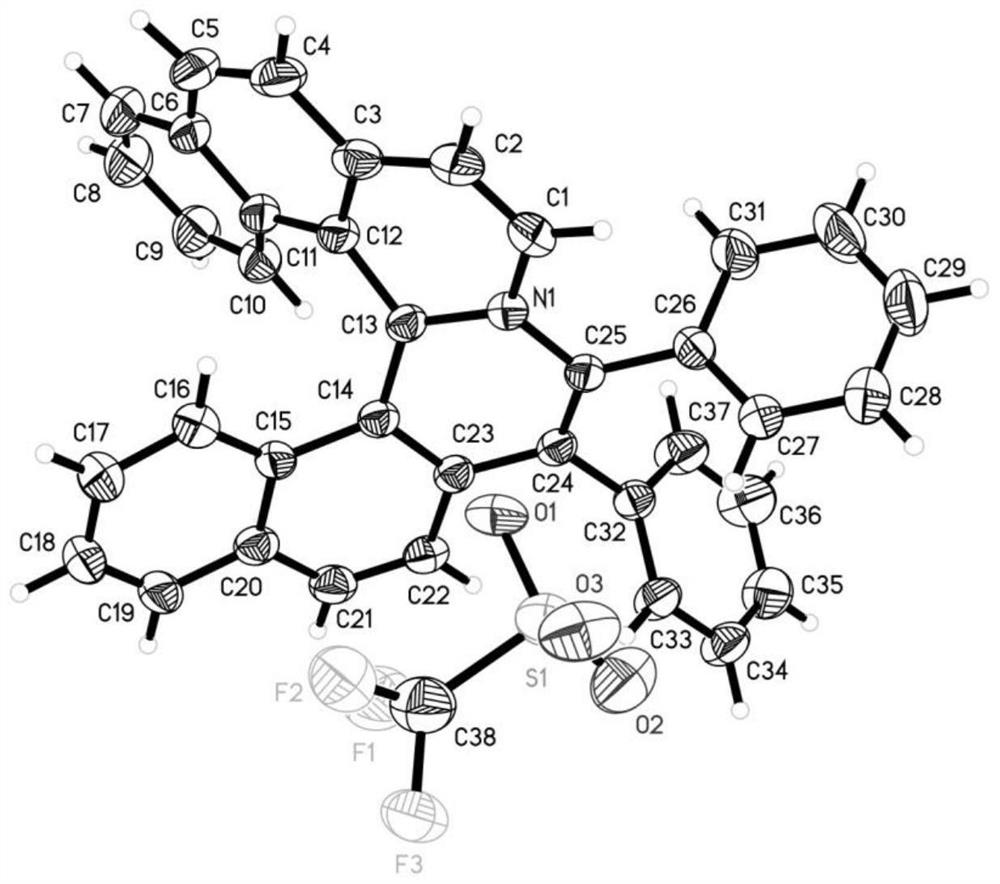
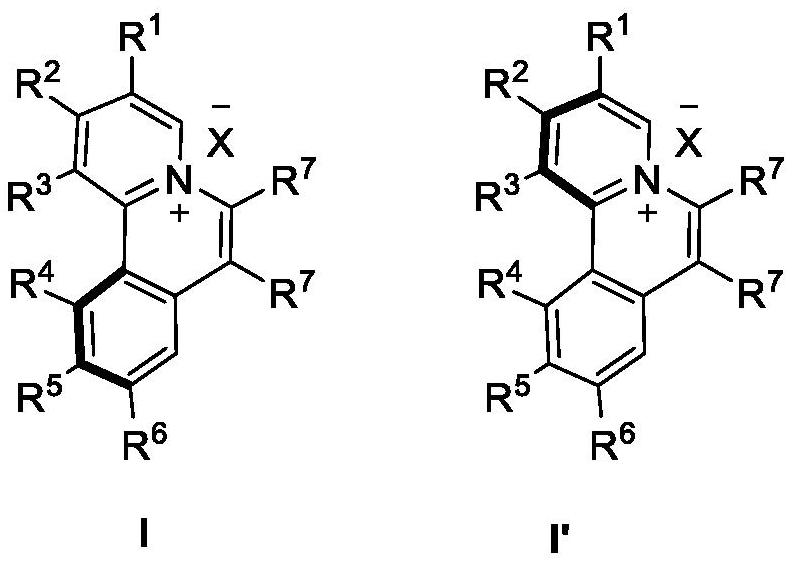
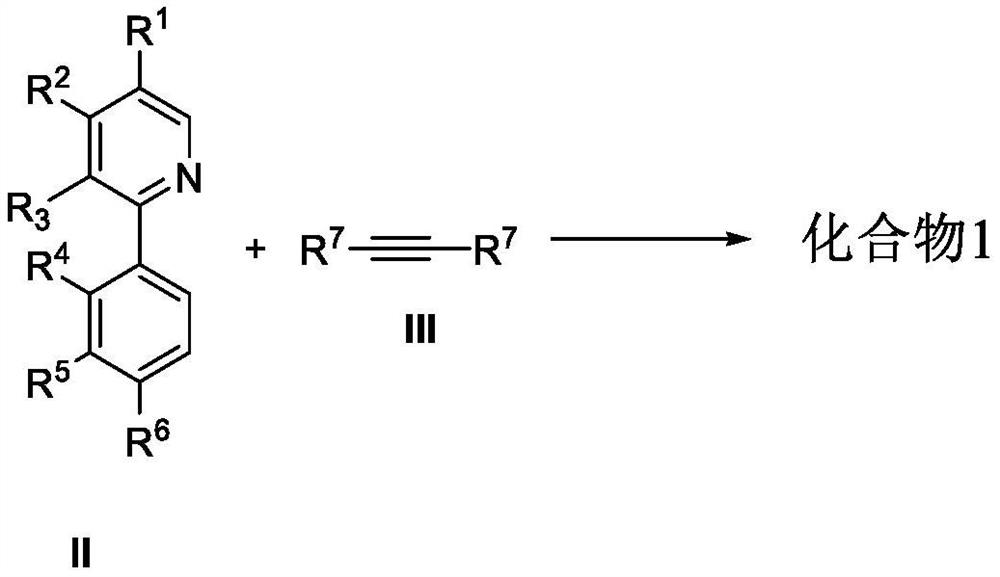Chiral azaspirene salt compound and preparation method thereof
A salt compound and compound technology are applied in the field of chiral azaspiroene salt compounds and their preparation, and can solve problems such as failure to successfully prepare chiral azaspiroene salt compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Embodiment 1 Synthesis of compound shown in formula I
[0176]
[0177] Under argon atmosphere, [SCpRh] (2.6mg, 0.005mmol), A2 (6.8mg, 0.02mmol), AgF (38.0mg, 0.30mmol), NaOTf (34.4mg, 0.20mmol), Compound II (0.10mmol) and the corresponding alkyne III (0.10mmol, 1.0equiv.), MeOH (1.0mL) and water (18mg, 1.0mmol) were then heated to 80°C for reaction. After the reaction, dilute with dichloromethane, filter with neutral alumina, remove the solvent residue under reduced pressure and separate by neutral alumina column chromatography to obtain the target product I (methanol / dichloromethane=1 / 20).
Embodiment 2
[0178] The synthesis of embodiment 2 compound I-1
[0179]
[0180] (62.5 mg, 99% yield, 91% ee). Analytical data: 1 H NMR (400MHz, CD 2 Cl 2 )δ8.62(d, J=7.1Hz, 1H), 8.50(d, J=8.5Hz, 1H), 8.35(d, J=8.8Hz, 1H), 8.22(d, J=7.1Hz, 1H) ,8.14(d,J=8.6Hz,1H),8.05(d,J=7.8Hz,1H),8.01(d,J=7.9Hz,1H),7.70(d,J=8.9Hz,1H),7.57 –7.29(m,13H),7.00–6.91(m,2H). 13 C NMR (101MHz, CD 2 Cl 2 )δ141.5,140.4,137.7,137.2,135.7,135.1,135.0,133.9,133.4,132.8,131.3,131.0,130.8,130.7,130.6,130.1,129.8,129.7,129.4,129.1,129.1,128.9,128.8,128.8,128.7 ,128.6,128.6,128.5,128.0,127.7,125.8,125.7,124.3,124.0,123.7,122.6,122.3. 19 F NMR (377MHz, CD 2 Cl 2 )δ-78.99. Chiralpak IB N-3column, 0.46cmI.D.×25cm L×3μm, 0.2% propylamine (adjust pH=3 with phosphoric acid) a.q. / acetonitrile, 50:50v / v, flow rate 0.4mL / min , detection wavelength λ=254nm,t R (major)=63.46min,t R (minor) = 67.91min.
Embodiment 3
[0181] The synthesis of embodiment 3 compound 1-2
[0182]
[0183] (63.8 mg, 97% yield, 93% ee). Analytical data: 1 H NMR (400MHz, CD 2 Cl 2 )δ8.64(d, J=7.1Hz, 1H), 8.51(d, J=7.1Hz, 1H), 8.45(d, J=8.5Hz, 1H), 8.37(d, J=8.9Hz, 1H) ,8.25(d,J=8.6Hz,1H),8.09–7.94(m,2H),7.67(d,J=8.8Hz,1H),7.66–7.58(m,1H),7.56–7.43(m,4H ),7.41(d,J=8.6Hz,1H),7.38–7.30(m,2H),7.28–7.19(m,2H),7.18–7.08(m,2H),6.99–6.87(m,2H). 13 C NMR (101MHz, CD 2 Cl 2 )δ164.5 (d, J=104.1Hz), 162.0 (d, J=100.6Hz), 141.8, 139.8, 137.6, 137.2, 135.1, 135.0, 134.9, 133.9 (d, J=8.8Hz), 133.4, 133.2 (d, J=8.7Hz),132.9,132.8,132.3(d,J=8.4Hz),129.9(d,J=3.7Hz),129.4,129.0,129.0,128.8,128.8,128.6(d,J=9.4 Hz), 128.0, 127.7, 126.7(d, J=3.7Hz), 125.9, 125.9, 124.4, 124.1, 123.6, 122.6, 122.1, 117.3(d, J=22.2Hz), 117.0(d, J=22.2Hz) ,116.0(d,J=21.8Hz),115.7(d,J=22.1Hz). 19 F NMR (377MHz, CD 2 Cl 2 )δ-79.04, -108.69, -112.52. Chiralpak IB N-3column, 0.46cm I.D.×25cm L×3μm, 0.2% propylamine (phosphoric acid to adjust p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com