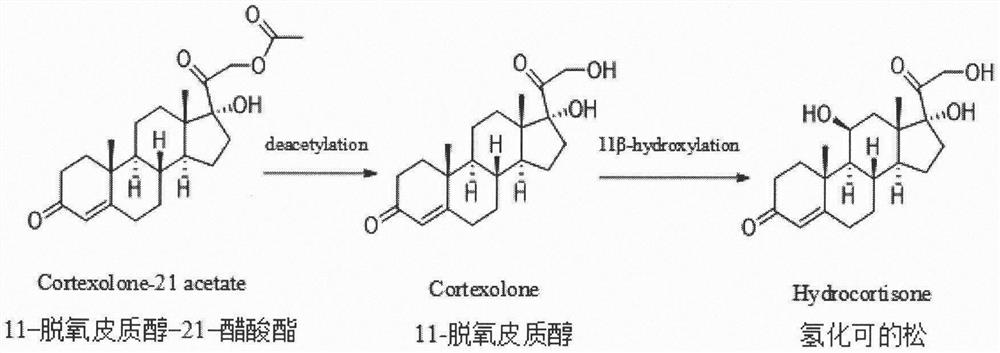
Construction of curvularia lunata steroid 11beta-hydroxylase CYP5103B6 mutant and application thereof
A mutant and hydroxylase technology, applied in the fields of genetic engineering and enzyme engineering, can solve the problems of low conversion rate, poor specificity of hydroxylation, and low feeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
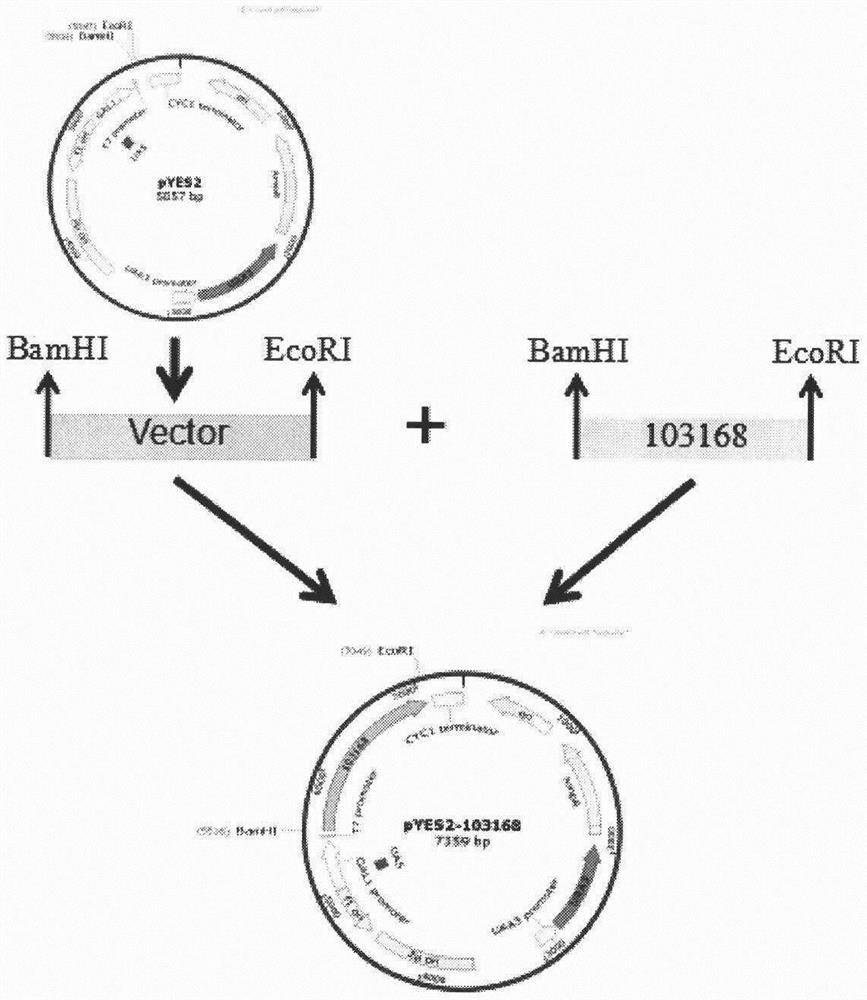
[0050] Example 1: Construction of pYES2-103168 recombinant Saccharomyces cerevisiae vector
[0051] 1. Amplification of CYP5103B6 gene 103168 of Curvularia lunata
[0052] Using Curvularia crescenae CYP5103B6 gene 103168 as a template, design upstream and downstream primers with BamHI and EcoRI restriction sites for PCR amplification. The primers were synthesized by Beijing Huada Gene Company.
[0053] F: CGGGATCCTACGTAATGGACCCTCAGACCGTTGGTC
[0054] R: GGAATTCTCAAACAACAACTCTCTTGAAGGCC
[0055] (1) PCR reaction system:
[0056]
[0057]
[0058] (2) PCR reaction conditions:
[0059] >55℃ program
[0060]
[0061] or <55℃ program
[0062]
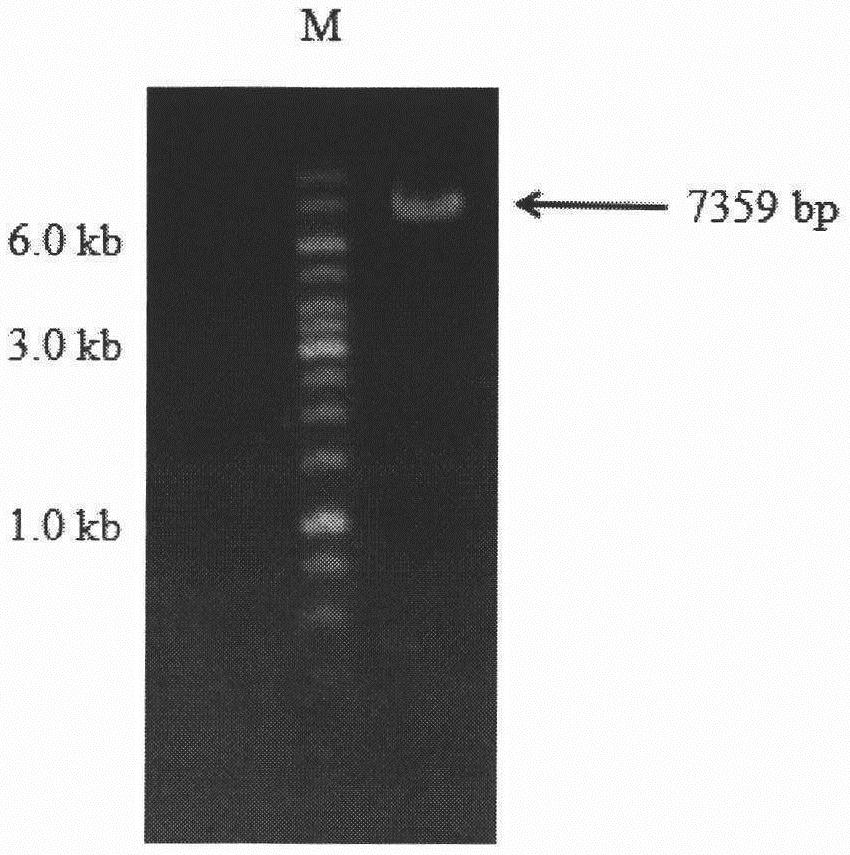
[0063] PCR products were verified to be correct by agarose gel electrophoresis, such as figure 2
[0064] 2. Purification and digestion of PCR products
[0065] The PCR product was digested with DpnI enzyme template, placed in a metal bath at 70°C for 20min (enzyme inactivation), and the PCR amplified fragment was recove...
Embodiment 2
[0072] Embodiment 2: Construction of mutants T291Y, M365A, A372F
[0073] (1) Site-directed mutagenesis technology amplifies the target fragment:
[0074] The plasmid pYES2-103168 was used as a template, and T291Y-F / R, M365A-F / R, and A372F-F / R were used as primers for polymerase chain reaction, and the primers were synthesized by Beijing Huada Gene Company. Primers for site-directed mutagenesis are as follows:
[0075] T291Y-F: CAAGTACCAGTTGTCTCTTATCTTCG
[0076] T291Y-R: TGGTACTTGGCCAATTCTTGGATAC
[0077] M365A-F: CCGCGACCAGTTTCACTAGAAGGG
[0078] M365A-R: GTCGCGGTTGGTCCGGTGAATCTC
[0079] A372F-F: GGTTCAGAAAAGGGAATCACCTTG
[0080] A372F-R: TTCTGAACCTTCTAGTGAAACTGGTC
[0081] Carry out site-directed mutagenesis according to the above-mentioned PCR amplification procedures to obtain site-directed mutation target vector fragments L1 (T291Y), L2 (M365A), and L3 (A372F). Take 2 μL of the PCR product and verify the band size by agarose gel electrophoresis. See Figure 5 sho...
Embodiment 3
[0084] Embodiment 3: Construction of mutant L4 (T291Y / M365A), L5 (T291Y / A372F), L6 (M365A / A372F)
[0085] 1. Amplify the target fragment by site-directed mutagenesis:
[0086] Using plasmid L1 (T291Y) as template and M365A-F / R as primers, polymerase chain reaction was used for site-directed mutagenesis to obtain site-directed mutation target vector fragment L4 (T291Y / M365A); using plasmid L1 (T291Y) as template, A372F -F / R as primers, use polymerase chain reaction for site-directed mutagenesis, and obtain site-directed mutation target vector fragment L5 (T291Y / A372F); use plasmid L2 (M365A) as template, A372F-F / R as primers, use polymerase The site-directed mutation was carried out by chain reaction to obtain the target vector fragment L6 (M365A / A372F) for site-directed mutation. Primers were synthesized by Beijing Huada Gene Company.
[0087] M365A-F: CCGCGACCAGTTTCACTAGAAGGG
[0088] M365A-R: GTCGCGGTTGGTCCGGTGAATCTC
[0089] A372F-F: GGTTCAGAAAAGGGAATCACCTTG
[0090] A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com