Preparation of spinal cord subacute combined degeneration disease model
A technology for subacute and degenerative diseases, applied in the field of medical biology, can solve the problems of insufficient vitamin B content, inability to complete vitamin B intake, and high cost of transgenic mice, reducing research costs, low prices, and increasing the speed of preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: The preparation of the spinal cord subacute combined degenerative disease model, the method comprises the following steps:
[0026] S1. Dilute 10 μl of hexachlorobutadiene into 490ul of edible oil, which is corn oil in this embodiment;
[0027] S2. According to the dose of 50-250 μl / mouse, the mice are given intragastric or intraperitoneal injection, and the mice are normally fed during the medication period;
[0028] S3. Perform intragastric or intraperitoneal injection again at an interval of 48 hours, for a total of three administrations;
[0029] S4. Test the mice 24 hours after the third administration, and observe whether there are clinical symptoms of subacute combined degeneration of the spinal cord:
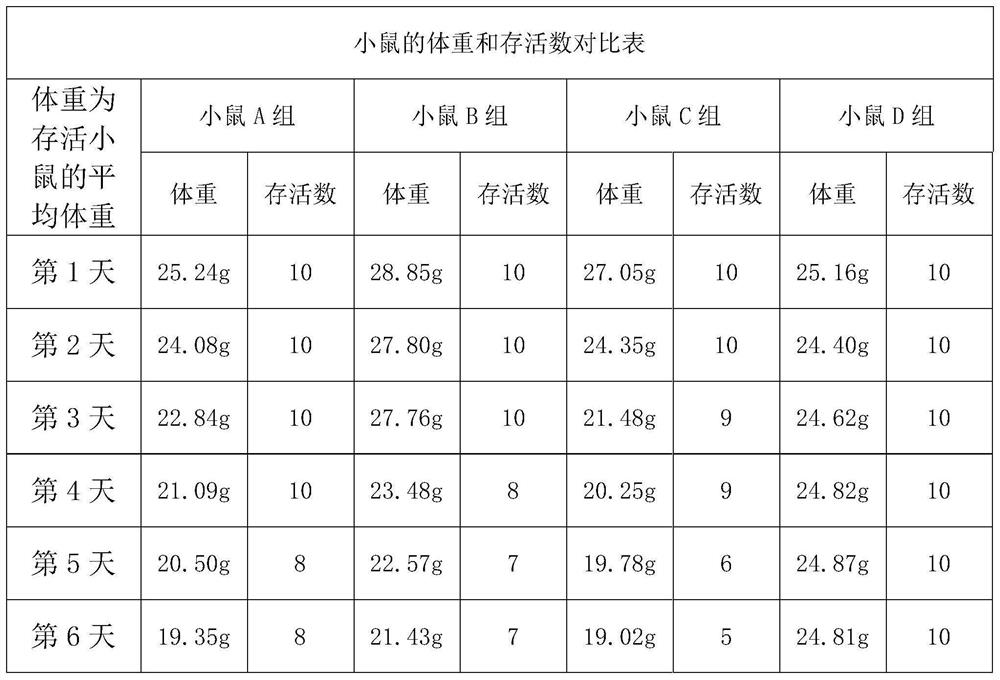
[0030] S4.1, the body weight of mice is tested and recorded every 24 hours during the medication period;
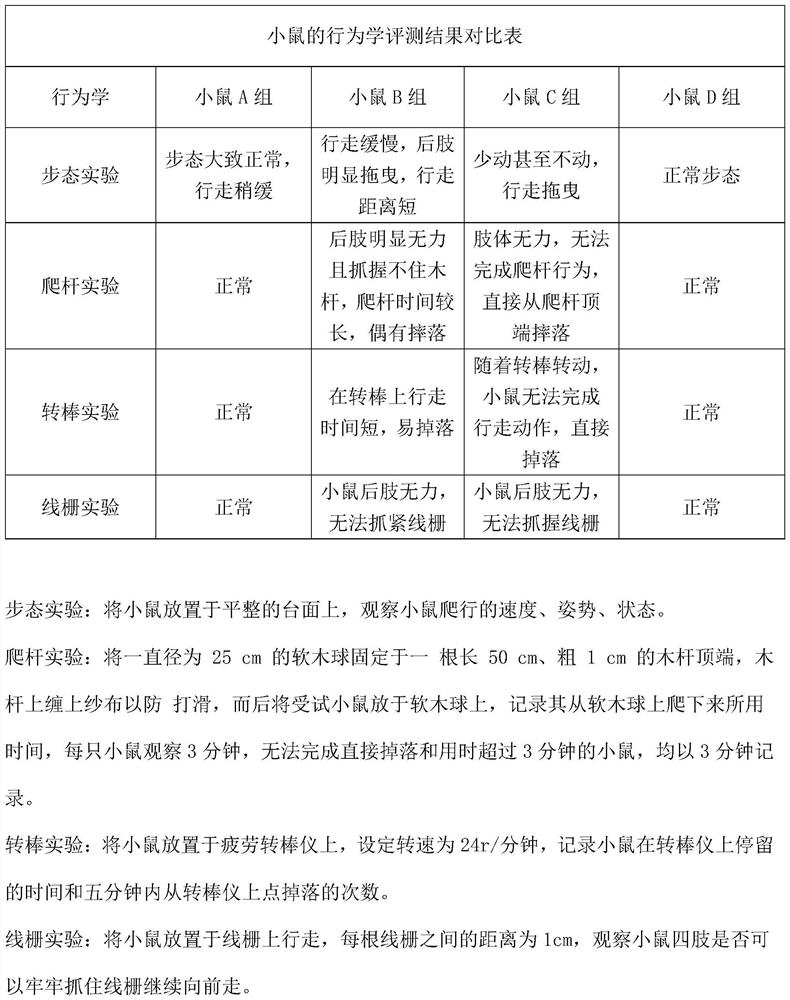
[0031] S4.2. Conduct behavioral evaluation on mice, including but not limited to: gait, pole climbing, rotarod and wire grid experiments;
...
Embodiment 2
[0033] Embodiment 2: Group A of test mice, a group of 10, carried out the following operations to it:
[0034] S1. Dilute 10 μl of hexachlorobutadiene into 490 μl of corn oil;
[0035] S2. According to the dose of 50 μl / mouse, the mice were given intragastric or intraperitoneal injection, and the mice were normally fed during the medication period;
[0036] S3. Perform intragastric or intraperitoneal injection again at an interval of 48 hours, for a total of three administrations;
[0037] S4. Test the mice 24 hours after the third administration, and observe whether there are clinical symptoms of subacute combined degeneration of the spinal cord:
[0038] S4.1, the body weight of mice is tested and recorded every 24 hours during the medication period;
[0039] S4.2. Conduct behavioral evaluation on mice, including but not limited to: gait, pole climbing, rotarod and wire grid experiments;
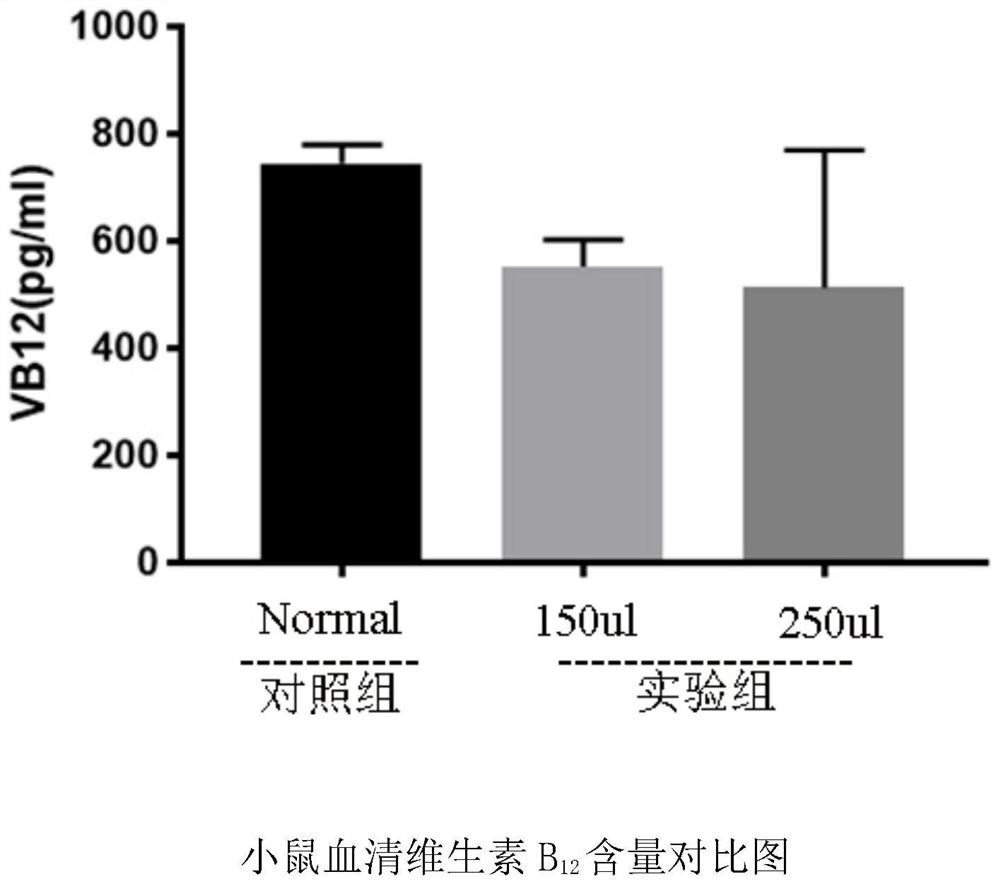
[0040] S4.3. Measurement of serum vitamin B in mice 12 content.
Embodiment 3
[0041] Embodiment 3: Group B of test mice, a group of 10, carried out the following operations to it:
[0042] S1. Dilute 10 μl of hexachlorobutadiene into 490 μl of corn oil;
[0043] S2. According to the dose of 150 μl / mouse, the mice were given intragastric or intraperitoneal injection, and the mice were fed normally during the medication period;
[0044] S3. Perform intragastric or intraperitoneal injection again at an interval of 48 hours, for a total of three administrations;
[0045] S4. Test the mice 24 hours after the third administration, and observe whether there are clinical symptoms of subacute combined degeneration of the spinal cord:
[0046] S4.1, the body weight of mice is tested and recorded every 24 hours during the medication period;
[0047] S4.2. Conduct behavioral evaluation on mice, including but not limited to: gait, pole climbing, rotarod and wire grid experiments;
[0048] S4.3. Measurement of serum vitamin B in mice 12 content.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com