Cabozantinib malate crystal form and preparation method and use thereof
A technology of crystal forms and acids, applied in the field of medicinal chemistry, can solve the problems of poor repeatability of the preparation method of crystal form M2, difficult process control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] The preparation method of embodiment 1 crystal form CSI:
[0141] Weighed 100.5 mg of compound I, then added 10.0 mL of a mixed solvent of acetic acid and toluene (1:1, v / v), and stirred magnetically at 50° C. until the solid was completely dissolved. The obtained clear solution was placed under the condition of 50° C. to stand for volatilization, and a solid sample was obtained after about 15 days.
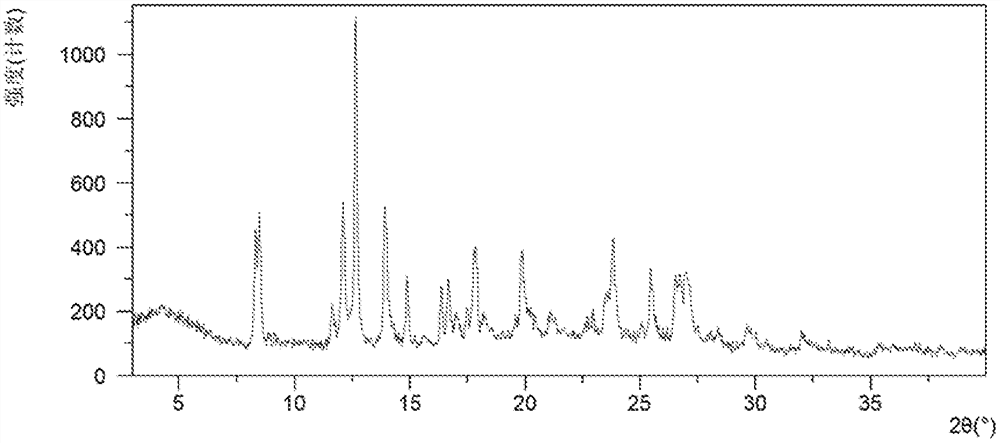
[0142] Gained solid carries out XRPD / TGA / DSC / 1 H NMR test characterization, its XRPD figure is attached figure 1 As shown, the XRPD data are shown in Table 1.
[0143] Its TGA, DSC and 1 H NMR characterization results are as follows:
[0144] TGA attached figure 2 As shown, when it is heated to 150°C, it has a mass loss of about 8.5%, corresponding to the removal of the acetic acid solvent during the heating process, and the crystal form CSI is an acetic acid solvate.
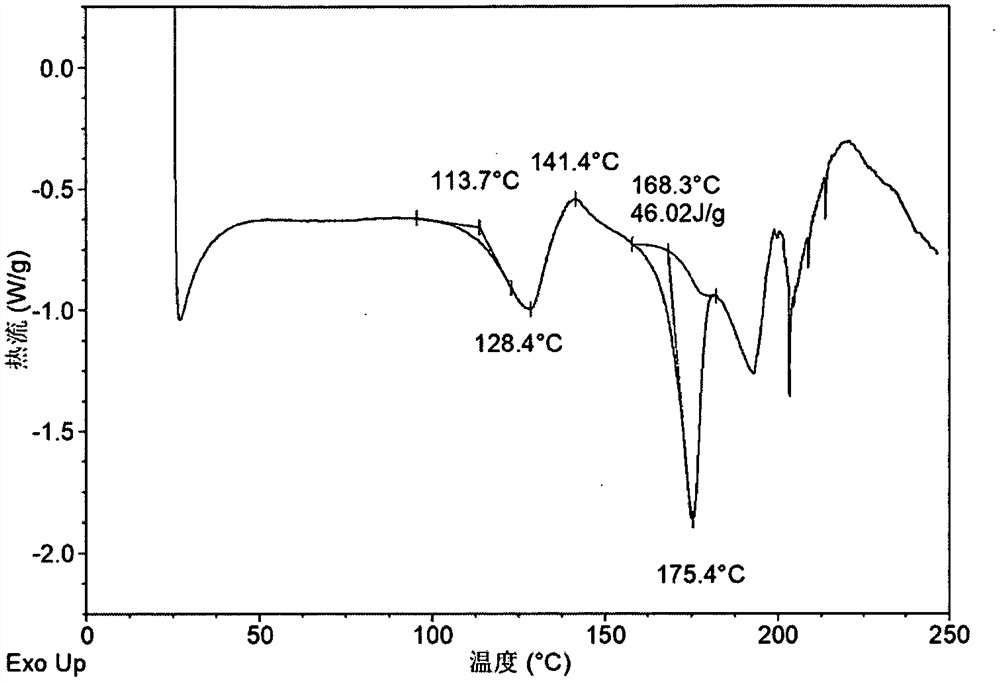
[0145] DSC attached image 3 As shown, an endothermic peak appears around 114 °C, followed by a...
Embodiment 2
[0150] The preparation method of embodiment 2 crystal form CSI
[0151] Weighed 2033.1 mg of compound I, then added 6.0 mL of acetic acid, and stirred magnetically at 50° C. until the solid was completely dissolved. After the solution was naturally cooled to room temperature, it was filtered to obtain a clear acetic acid solution. At room temperature, add toluene to the clear solution while stirring, 1.0 mL each time, 20.0 mL in total. The resulting suspension was transferred to 5°C and stirring was continued for about 24 hours. A sample of the precipitated solid was isolated.
[0152] Characterized by XRPD, the crystal form of the obtained solid is CSI, and the corresponding XRPD pattern and XRPD data are shown in the appendix Figure 5 and Table 2.
[0153] Table 2
[0154]
[0155]
Embodiment 3
[0156] The preparation of embodiment 3 crystal form CSIII
[0157] Weigh 5.1 g of Compound I solid and dissolve it in 25.0 mL of acetic acid, stir at 100°C until the solid is completely dissolved, and add 25.0 mL of toluene after the solution is cooled to room temperature. Filtered at room temperature to obtain a clear solution, which was transferred to a reaction kettle and continued to cool to 0°C. Then 52.1 mg of seed crystals were added, mechanically stirred and matured for 1.5 hours, and then 50.0 mL of isopropyl acetate was added, and the solid was separated after stirring for 20 hours. The separated solid was transferred to 100.5 mL of toluene and water (200:1, v / v) and slurried for about 2 minutes, and then the solid was separated.
[0158] Characterized by XRPD, the crystal form of the obtained solid is CSIII, and its XRPD pattern is shown in the attached Figure 6 As shown, the XRPD data are shown in Table 3.
[0159] table 3
[0160]
[0161]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com