Immunoglobulin-binding protein, and affinity carrier using same
A technology of immunoglobulin and protein, which is applied in the field of immunoglobulin-binding protein, and can solve problems such as low alkali resistance and reduced antibody binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0322] Hereinafter, an Example is given and this invention is demonstrated more concretely. In addition, the following description generally shows the aspect of this invention, and unless otherwise indicated, this invention is not limited to these descriptions.
reference example 1
[0323] Reference Example 1: Synthesis of Porous Particles
[0324] (1) Add 3.58 g of polyvinyl alcohol (PVA-217 manufactured by Kuraray Co., Ltd.) to 360 g of pure water, heat and stir to dissolve the polyvinyl alcohol, and after cooling, add sodium lauryl sulfate (manufactured by Wako Pure Chemical Industries) 0.36 g, 0.36 g of sodium sulfate (manufactured by Wako Pure Chemical Industries) and 0.18 g of sodium nitrite (manufactured by Wako Pure Chemical Industries) were stirred to prepare an aqueous solution S.
[0325] (2) Dissolve a monomer composition composed of 12.00 g of glycidyl methacrylate (manufactured by Mitsubishi Chemical Corporation) and 1.33 g of divinylbenzene (manufactured by Nippon Steel Chemical Co., Ltd.) in diisobutyl ketone (Mitsui Chemicals Co., Ltd. Chemical Co., Ltd.) 24.43 g to prepare a monomer solution.
[0326] (3) Put the whole amount of the aqueous solution S obtained in (1) into a separable flask, install a thermometer, a stirring blade and a ...
Embodiment 1
[0328] Example 1: Production of immunoglobulin-binding proteins (PrAt-0-32)
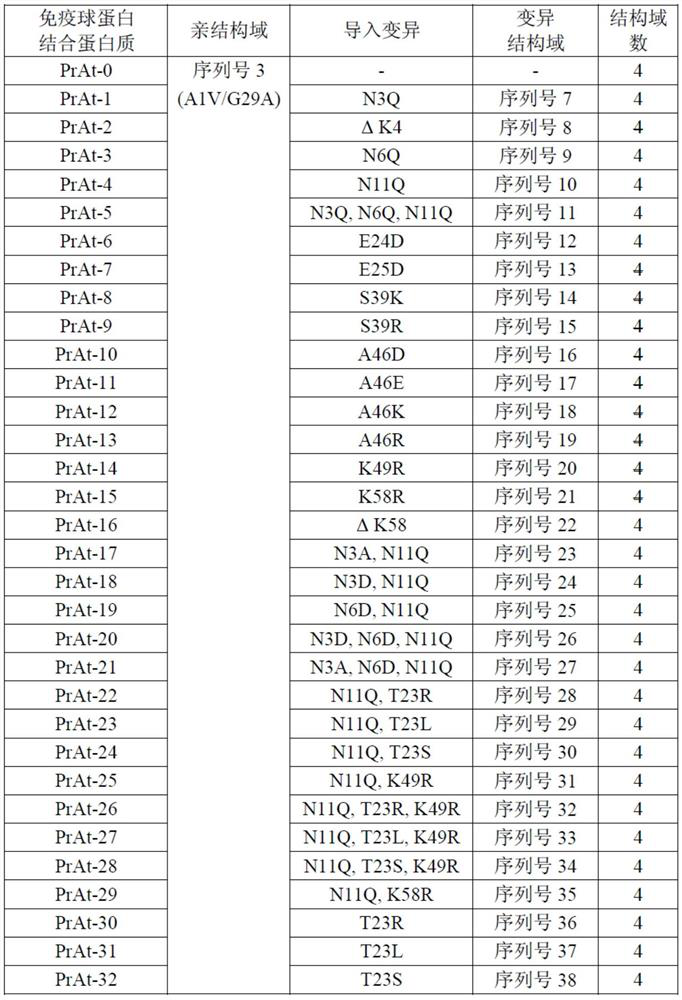
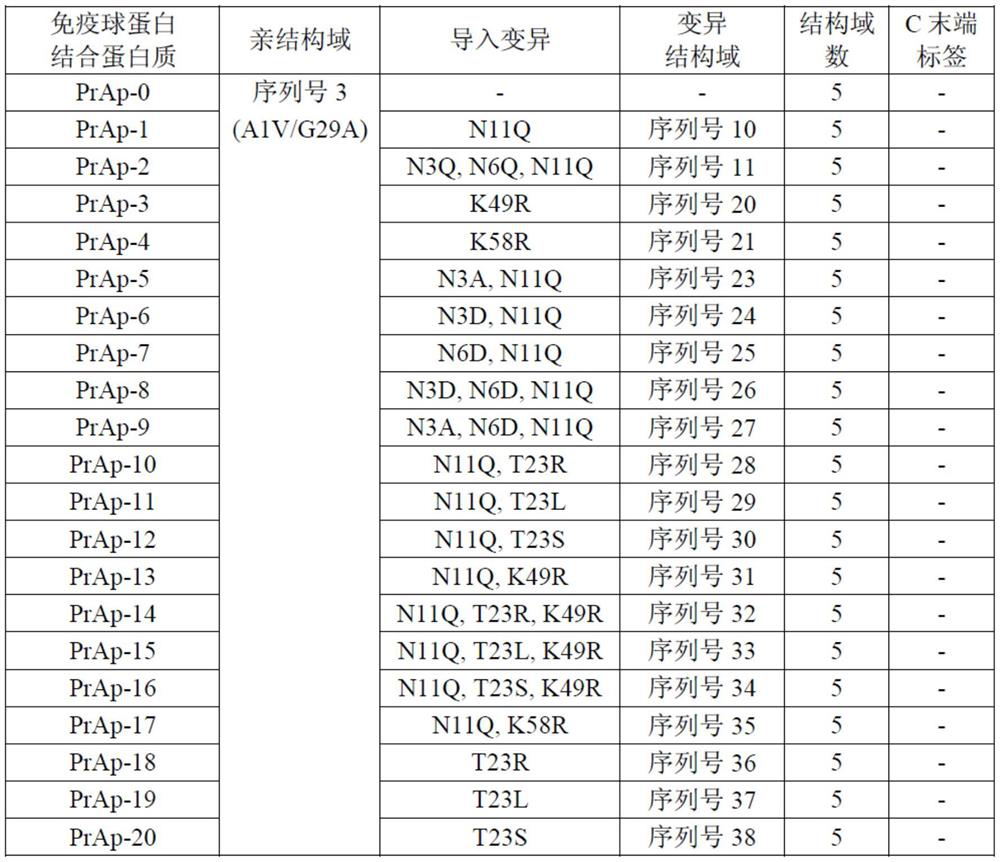
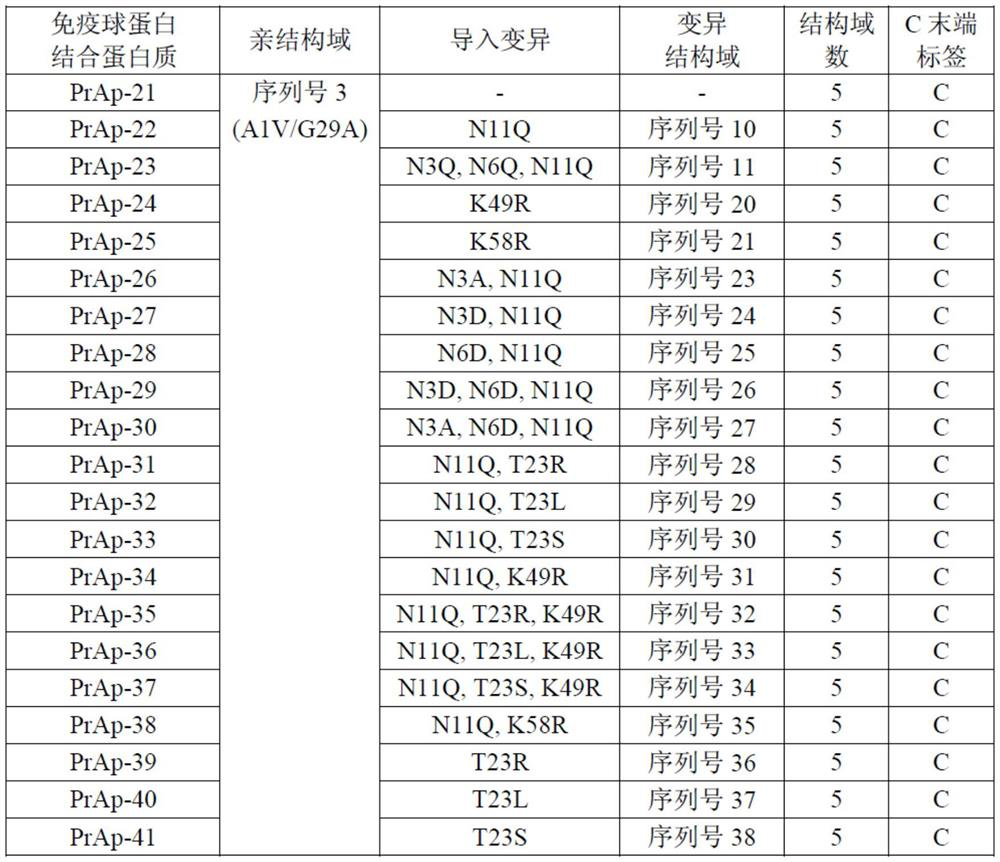
[0329] Immunoglobulin-binding proteins PrAt-0-32 were obtained. PrAt-0 is a homotetramer in which an A1V / G29A variant (alkali-resistant variant; non-patent document 1) comprising the C domain of protein A (SEQ ID NO. 3) is connected in series by peptide bonds Immunoglobulin binding protein. PrAt-1 to 32 are variants obtained by introducing the mutations described in Table 1 into the respective immunoglobulin-binding domains of PrAt-0.
[0330] [Table 1]
[0331]
[0332]Expression and purification of PrAt-0 to 32 were carried out as follows. Escherichia coli BL21(DE3) (manufactured by New England Biolabs) was transformed with plasmids encoding PrAt-0 to 32, and the obtained transformants were cultured in a nutrient-rich medium at 37° C. until logarithmic growth phase. Then, the target protein was expressed by adding isopropyl-β-thiogalactopyranoside (manufactured by Wako Pure Chemical Industri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com