A kind of recombinant river crab esflol protein with binding activity to chh and its application
A technology that combines activity and protein, applied in the biological field, can solve problems such as major disputes, and achieve the effect of realizing profits, maximizing and shortening the breeding cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Prokaryotic Expression and Purification of Esflol in Eriocheir sinensis
[0039] The prokaryotic expression vector is PET-30 vector, and the expression strain is Rosetta strain. Etaq enzyme, DNA markerDL2000, pMD19-T vector, Nde I, EcorⅠ, DNA Ligation kit were all purchased from TaKaRa Company; agarose, SanPrep column type DNA mini-extraction kit, SanPrep column type plasmid DNA mini-extraction kit , LB solid medium, LB liquid medium, isopropanol, isopropyl-B-D-thiogalactoside (IPTG), glycine, EDTA, SDS, TEMED, Acrylamide (40%), ammonium persulfate, Coomassie Reagents such as blue R-250, bromophenol blue, and Tris were purchased from Shanghai Sangon Bioengineering Co., Ltd.; protein markers were purchased from Fermentas; other reagents were of domestic analytical grade.
[0040] 1.1 Construction of expression vector
[0041] (1) Design PCR primers
[0042] F1: 5'-AATTCCATATGATTCCCATTTTCATCA-3';
[0043] R1: 5'-AATTCTCAGTGGTGGTGGTGGTGGTGTGCTACACGGAGGTT-3',
[0044] ...
Embodiment 2
[0075] Pull-Down experiment of Esflol and CHH
[0076] (1) The prokaryotic expression system constructed by using the constructed Esflol and CHH was expressed, purified, and refolded to obtain two target proteins dissolved in TBS solution, which were stored in a -80°C ultra-low temperature refrigerator for later use.
[0077] (2) Take out the protein sample stored in the ultra-low temperature refrigerator, melt it at room temperature, detect the protein concentration with Nanodrop, and place it on ice for later use;
[0078] (3) Assemble the three sets of prepacked columns and recovery tubes, and mark them as T1, C1, and C2 respectively;
[0079] (4) Prepare 30ml wash solution: mix TBS solution and Pierce Lysis Buffer according to the ratio of 1:1-1:3, and add 3-6M imidazole Stock Solution, so that the final concentration of imidazole in the wash solution is 10mM;
[0080] (5) Mix the HisPur Cobalt Resin filler with a vortexer, and pour 50 μL of the fully mixed filler into ea...
Embodiment 3
[0104] result
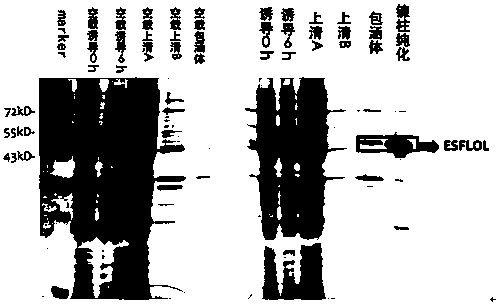
[0105] 1. Prokaryotic expression of Esflol in Chinese mitten crab
[0106] 1.1 Construction of Esflol prokaryotic expression vector
[0107] In this experiment, the PET30-Esflol expression vector was successfully constructed, and the recombinant Esflol protein was successfully obtained through the prokaryotic expression of the Rosetta strain. In the process of constructing prokaryotic expression system, the selection of expression strain is very important. Esflol has a relatively large molecular weight and is a membrane protein. It is difficult to express and obtain it through prokaryotic. The codon preference of eukaryotic cells is different from that of prokaryotic systems. Therefore, when using prokaryotic systems to express eukaryotic genes, some codons in eukaryotic genes may be rare codons for prokaryotic cells, resulting in expression efficiency and expression levels are very low. Esflol protein has such a rare codon. Therefore, the Rosetta strain w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com