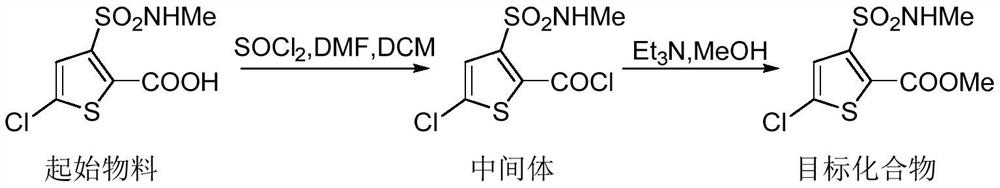
Synthesis method of lornoxicam intermediate 5-chloro-3-methylsulfonamide thiophene-2-carboxylic acid methyl ester
A technology of methylsulfonamide and methyl carboxylate, which is applied in the field of drug synthesis, can solve problems such as pollution and unenvironmental protection of the process, and achieve the effect of reducing pollution and environmental protection of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
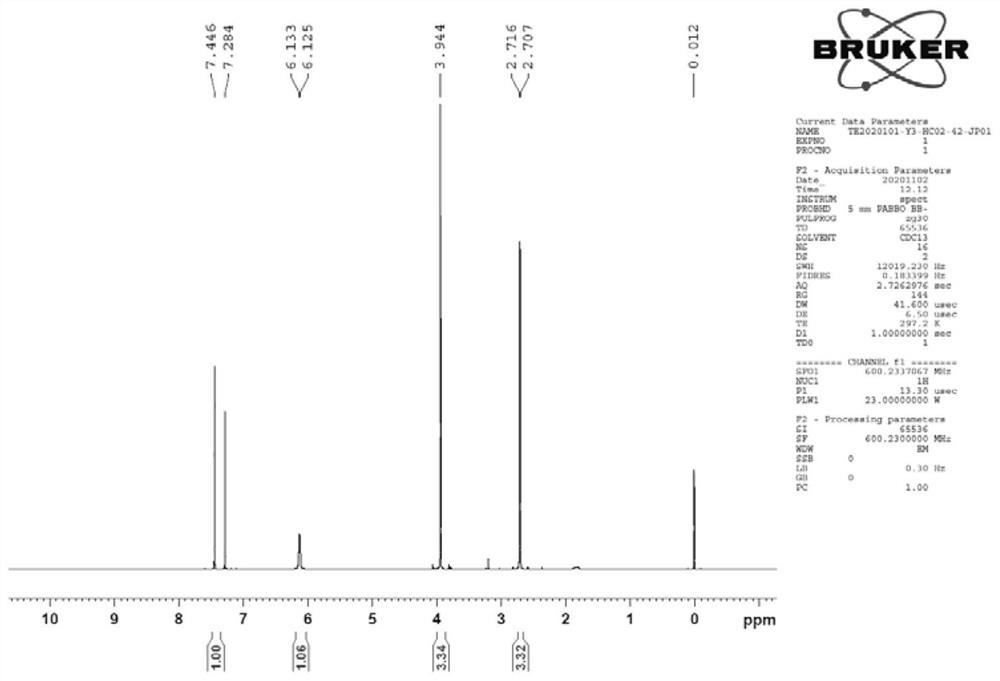
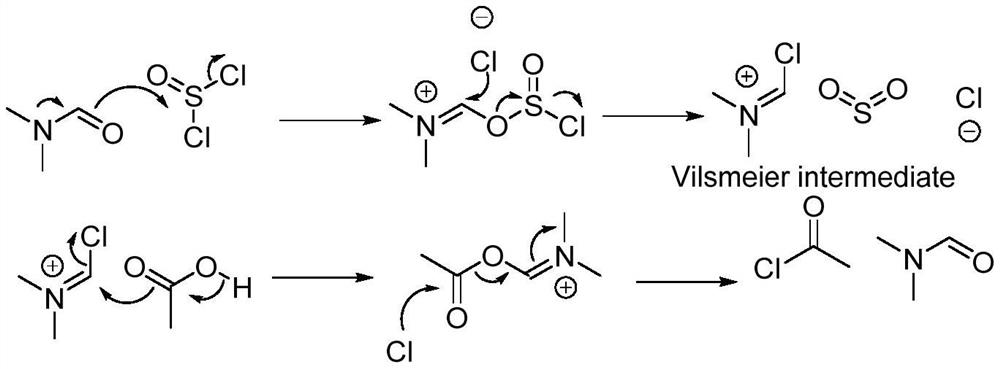
[0042] Under the protection of nitrogen, add 250ml of dichloromethane and 50.0g of 5-chloro-3-methylsulfonamide thiophene-2-carboxylic acid into a 100mL four-necked bottle, and add N,N-dimethylformaldehyde at a temperature of 0-5°C Amide 14.0g, temperature control 0-5°C, add 46.5g of thionyl chloride dropwise, after the dropwise addition, slowly raise the temperature to 45°C and reflux, keep warm and reflux for 4 hours, control the temperature at 45-50°C, add dropwise 150ml of methanol, and then 50ml of triethylamine was added to continue the incubation and reflux reaction for 4 hours.
[0043] After the solvent was removed under reduced pressure at a temperature of 30-35°C, the crude product of methyl 5-chloro-3-methylsulfonamide thiophene-2-carboxylate was obtained, which was transferred to 5-chloro-3-methylsulfonamide thiophene-2-carboxylate Add 150ml of methanol to the crude methyl ester and heat up to 50°C until the system is completely dissolved. After 1 hour of heat pre...
Embodiment 2
[0045] Under the protection of nitrogen, add 250ml of dichloromethane and 50.0g of 5-chloro-3-methylsulfonamide thiophene-2-carboxylic acid into a 100mL four-necked bottle, and add N,N-dimethylformaldehyde at a temperature of 0-5°C Amide 14.1g, temperature control 0-5°C, add 69.8g of thionyl chloride dropwise, after the dropwise addition, slowly raise the temperature to 50°C and reflux, keep warm and reflux for 2 hours, control the temperature at 45-50°C, add dropwise 150ml of methanol, and then 50ml of triethylamine was added to continue the incubation and reflux reaction for 2 hours.
[0046]After the solvent was removed under reduced pressure at a temperature of 30-35°C, the crude product of methyl 5-chloro-3-methylsulfonamidethiophene-2-carboxylate was obtained, which was transferred to 5-chloro-3-methylsulfonamidethiophene-2-carboxylate Add 150ml of methanol to the crude methyl ester and raise the temperature to 40°C until the system is completely dissolved. After 1 hour ...
Embodiment 3
[0048] Under nitrogen protection, add 250ml of dichloromethane and 50.0g of 5-chloro-3-methylsulfonamide thiophene-2-carboxylic acid into a 100mL four-necked bottle, and add N,N-dimethylformaldehyde at a temperature of 0-5°C Amide 14.2g, temperature control 0-5°C, add 46.7g of thionyl chloride dropwise, after the dropwise addition, slowly raise the temperature to 48°C to reflux, keep warm and reflux for 4 hours, control the temperature to 45-50°C, add dropwise 150ml of methanol, and then Add 75ml triethylamine and continue to insulate and reflux the reaction for 6 hours.
[0049] After the solvent was removed under reduced pressure at a temperature of 30-35°C, the crude product of methyl 5-chloro-3-methylsulfonamidethiophene-2-carboxylate was obtained, which was transferred to 5-chloro-3-methylsulfonamidethiophene-2-carboxylate Add 150ml of methanol to the crude methyl ester and raise the temperature to 60°C until the system is completely dissolved. After 1 hour of heat preser...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com