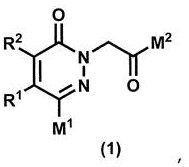
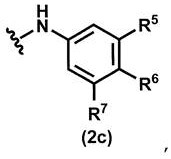
Pyridazinone derivative
A compound and optional technology, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, muscular system diseases, etc., can solve the problems of prolonging the PR interval of electrocardiogram and QRS width, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0925] N-(4-cyanophenyl)-2-[3-(4-methylpiperidin-1-yl)-6-oxopyridazin-1(6H)-yl]acetamide
[0926]
Embodiment 2
[0930]N-(4-cyanophenyl)-2-[3-(4,4-dimethylpiperidin-1-yl)-6-oxopyridazin-1(6H)-yl]acetamide
[0931]
[0932] The compound of Reference Example 3 (60 mg), 4,4-dimethylpiperidine hydrochloride (93 mg) and diisopropylethylamine (1 mL) were dissolved in dimethylacetamide (0.5 mL) The solution was stirred at 150 °C for 11 h. After the reaction was completed, water was added thereto, and the mixture was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by reverse phase HPLC (eluent; 0.035% trifluoroacetic acid in acetonitrile / water) and then by amino silica gel column chromatography (solvent; chloroform:methanol=99:1, then 93: 7) Purification to give the title compound (22 mg).
[0933] 1 H-NMR (400 MHz, CDCl 3 ) δ : 9.78 (s, 1H), 7.65 (d, J = 8.5 Hz, 2H),7.57 (d, J = 8.5 Hz, 2H), 7.25 (d, J = 9.8 Hz, 1H), 6.94 (d, J = 10.4 Hz,1H), 4.84 (s, 2H), 3.32-3.28 (m, ...
Embodiment 3-36
[0935] According to the method of Example 1 or 2 and common reaction conditions, the compounds of Examples 3-36 were obtained using the corresponding material compounds.
[0936]
[0937]
[0938]
[0939]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com