Animal-derived-component-free passaging method for passaging of stem cells
A technology of animal origin and stem cells, applied in the field of biomedicine, can solve the problems of prolonging the digestion process, affecting the state of stem cell culture, and uneven digestion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
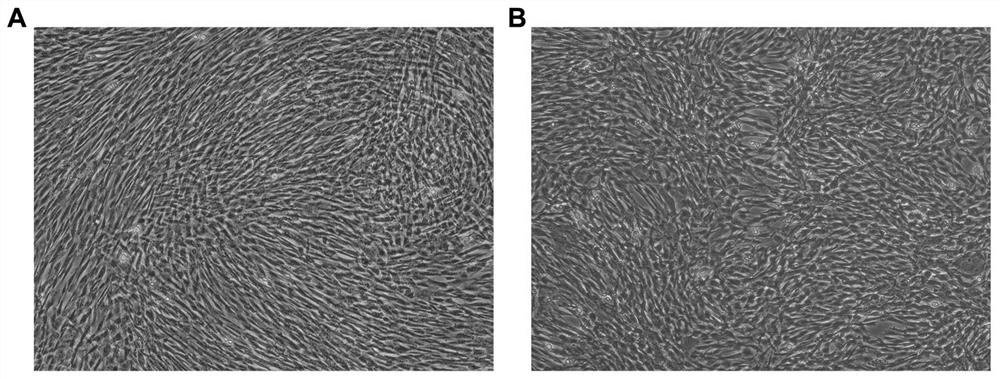
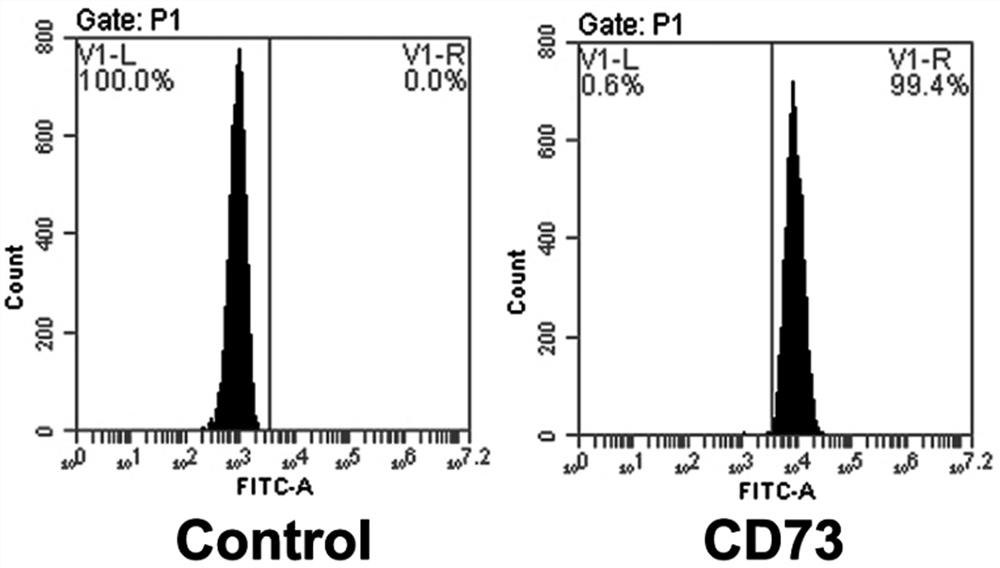
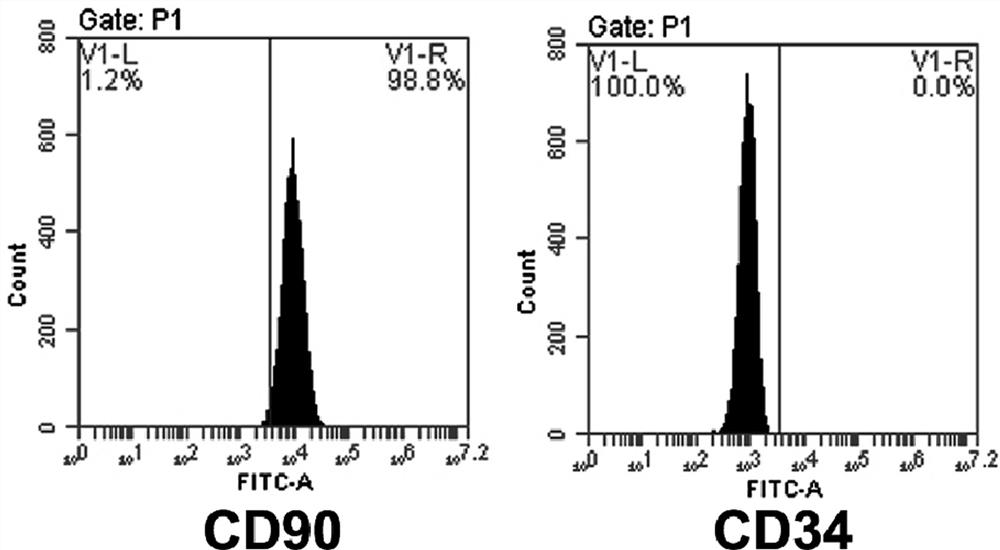
[0035]Example 1 Extract the human amniotic membrane, umbilical cord serum, routinely cultured to the third generation, and cell identification was carried out.
[0036]I. Materials and methods.
[0037]1, material.
[0038]DMEM / F12 medium (Hyclone, United States); ElitegroTM-Advanced (GMP Degree); PBS buffer (Biological Industries, Israel); collagenase IV (Thermofisher, USA); MESENCULTTM Osteogenic DiffERENTIATION KIT (HUMAN) (STEMCELL, USA); MESENCULTTMAdipogenic DiffERENTIATION KIT (HUMAN) (STEMCELL, USA);
[0039]MESENCULTTM-ACF CHONDROGENIC DIFFERENTIATION KIT (STEMCELL, USA); Alizarin Red S (Sedae Biology, China); Oil Red O (Fair Biology, China); Alcian Blue (Sedai Biology, China); CD34 FITC direct target antibody (BioLegend , United States); CD73FITC direct target antibody (BioLegend, United States); CD90FITC direct target antibody (Biolegend, US); HLA-DRFITC direct antibody (BioLegend, USA).
[0040]No animal source component digestion: The concentration unit Mg / L is calculated from the...
Embodiment 2
[0053]Type 3 generations of amnunopions, umbilical-cord metamorphosis stem cells in 75cm2Culture in the culture flask, the treatment of 80-90%.
[0054]I. Materials and methods.
[0055]1, material.
[0056]DMEM / F12 medium (Hyclone, United States); ElitegroTM-Advanced (GMP Degree) (Dako, China); PBS buffer (Biological Industries, Israel).
[0057]2, method.
[0058]Culture of Hamscs Cells: Take the third generation of amniotic membranes under the culture of animals source components, umbilical tape, tanneous stem cells, according to 5 × 104 / cm2Turn on 75cm2The bottle was cultured, and the cell cell density was observed after inoculation, and the cell convergence was 80-90%.
Embodiment 3
[0059]Example 3 Apply an animal-free component digestible liquid, digestive termination liquid, a human amniocenger, umbilical-cord metamorphosis stem cells.
[0060]I. Materials and methods.
[0061]1, material.
[0062]PBS buffer (Biological Industries, Israel); mild digestive enzyme (Friend Kang, China); no animal source component digestion; digestive termination; DMEM / F12 medium (Hyclone, United States); ElitegroTM-Advanced (GMPDEGREE) (Dako is China).
[0063]No animal source component digestion: The concentration unit Mg / L is calculated from the following concentrations of components: EDTA-2NA100, recombinant human trypsin 50, potassium hydrogen phosphate 60, potassium chloride 400, sodium bicarbonate 350, Sodium chloride 8000.
[0064]Digestive Termination: Meeting in a concentration unit, is comprised of the following concentrations of components: sodium chloride 8000, dihydrate sodium hydrogen sodium hydrogen phosphate 125, potassium chloride 400, potassium phosphate 60, magnesium sul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com