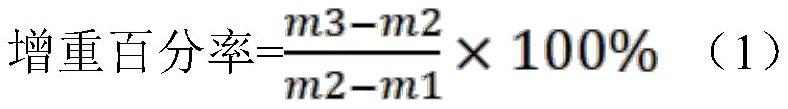Method for delivering levosalbutamol to lung in inhaled dry powder
A technology of albuterol and albuterol sulfate, which is applied in the field of medicine and achieves the effect of good hydrodynamic characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] Example 1: Hygroscopicity, angle of repose and powder diffraction experiments of three salt forms of levosalbutamine
[0013] Moisture test:
[0014] First, put the washed and dried flat weighing bottle in an artificial climate chamber (setting temperature: 25°C±1°C, relative humidity: 80%±2%) for 24 hours, and accurately weigh m1; The solid raw material medicine of quality standard is used as test product, and test product is placed in above-mentioned weighing bottle and evenly tiled, and test product thickness is generally about 1mm, and accurately weighs m2; Then weigh bottle exposure, and Place the bottle cap under the same constant temperature and humidity conditions for 24 hours, close the cap of the weighing bottle, and accurately weigh m3 after taking it out.
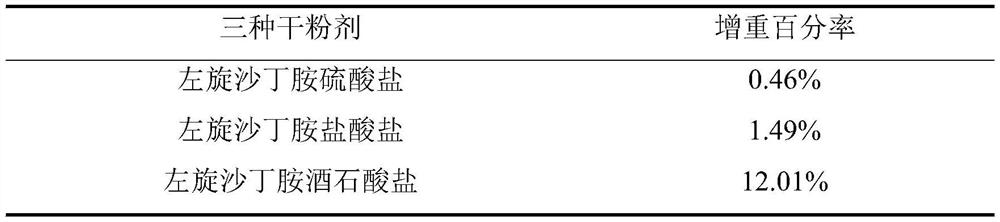
[0015] Calculate the weight gain percentage according to the following formula (1), and the results are shown in Table 1.
[0016]
[0017] Table 1
[0018]
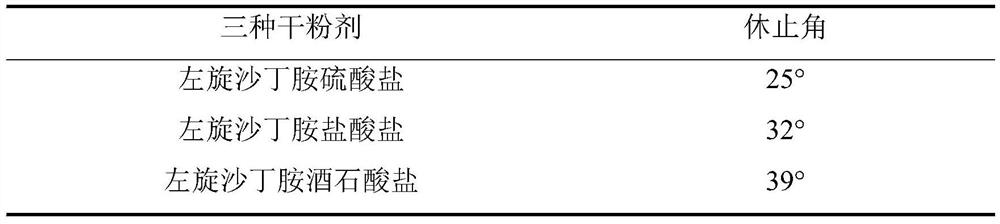
[0019] Angle of repose experiment
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com