Tellurium-containing compound, method for preparing tellurium gold nanoparticles and application of tellurium gold nanoparticles in preparation of antitumor drugs
A tellurium compound, 2-te technology, applied in the field of nano-biomedicine, can solve the problems of harsh storage conditions, cumbersome synthesis steps, toxic and side effects, etc., and achieve good thermodynamic stability, broad application prospects, and low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
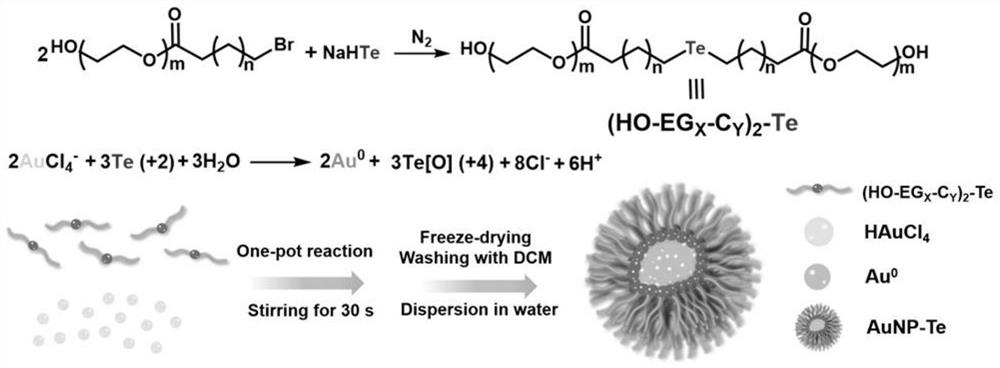
[0053] Embodiment 1: based on (HO-EG 6 -C 6 ) 2 Preparation of gold tellurium nanoparticles of -Te (bis(17-hydroxy-3,6,9,12,15-pentaoxoacetyl) 4,4'-telluryl dihexanoate) and chloroauric acid and its preparation Application in antineoplastic drugs
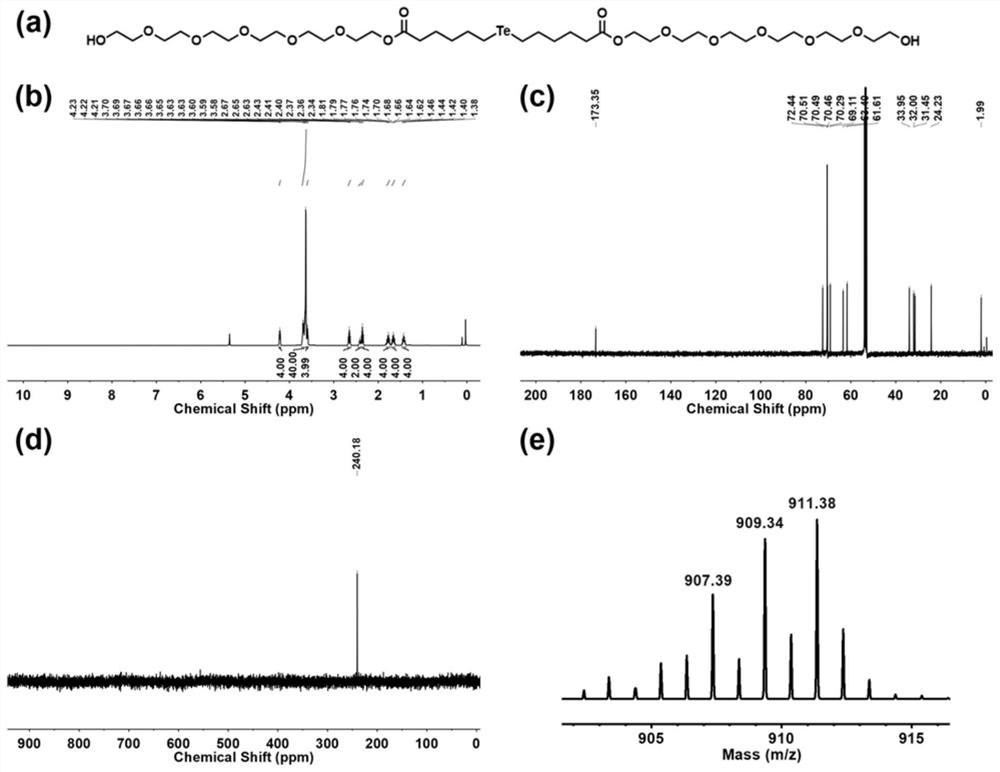
[0054] (1) 88.7mg (HO-EG 6 -C 6 ) 2 -Te was dissolved in 1 mL of deionized water to obtain component A, and 9.6 mg of chloroauric acid was dissolved in 1 mL of deionized water to obtain component B. Fully mix component A and component B in a 5mL reaction bottle to obtain a uniform mixed system. The above mixed system was lyophilized, rinsed three times with 2 mL of dichloromethane, and dried with nitrogen to obtain gold tellurium nanoparticles. where (HO-EG 6 -C 6 ) 2 The synthesis process of -Te molecule is as follows:
[0055] Dissolve 5.64g of hexaethylene glycol in a round-bottomed flask containing 100mL of dichloromethane, and add 4.27g of 6-bromohexanoyl chloride dropwise to the round-bottomed flask through a consta...
Embodiment 2
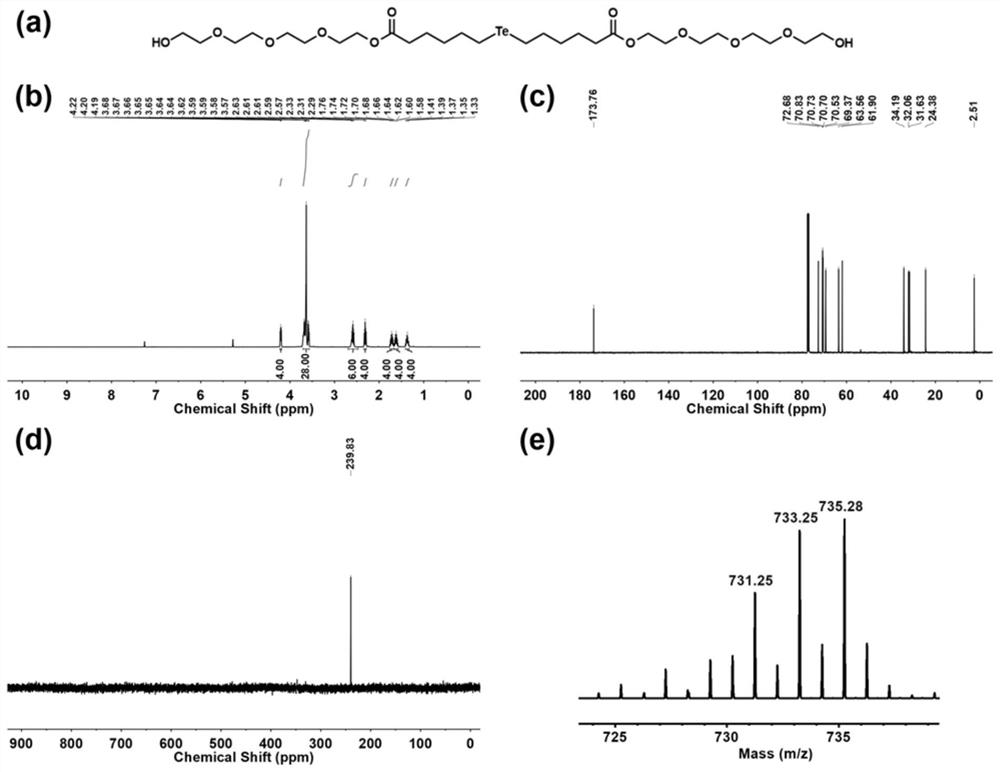
[0065] Embodiment 2: based on (HO-EG 4 -C 6 ) 2 -Te(bis(2-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)ethyl)4,4'-telluryl dihexanoate) with tellurium of chloroauric acid Preparation of gold nanoparticles and its application in the preparation of antitumor drugs
[0066] The difference between this embodiment and Example 1 lies in the preparation of gold tellurium nanoparticles, and all the other steps are the same as in Example 1, specifically:
[0067] 142.0mg (HO-EG 4 -C 6 ) 2-Te was dissolved in 1 mL of ethylene glycol to obtain component A, and 2.6 mg of chloroauric acid was dissolved in 1 mL of dimethyl sulfoxide to obtain component B. Fully mix component A and component B in a 5mL reaction bottle to obtain a uniform mixed system. The above mixed system was dried in a vacuum oven, rinsed three times with 2 mL of tetrahydrofuran, and dried in a vacuum oven to obtain gold tellurium nanoparticles. where (HO-EG 4 -C 6 ) 2 The synthesis process of -Te molecule is as follow...
Embodiment 3
[0069] Embodiment 3: based on (HO-EG 2 -C 6 ) 2 Preparation of gold tellurium nanoparticles of -Te (bis(2-(2-hydroxyethoxy)ethyl) 6,6'-telluryl dihexanoate) and chloroauric acid and its application in the preparation of antitumor drugs
[0070] The difference between this embodiment and Example 1 lies in the preparation of gold tellurium nanoparticles, and all the other steps are the same as in Example 1, specifically:
[0071] 17.8mg (HO-EG 2 -C 6 ) 2 -Te was dissolved in 1 mL of methanol to obtain component A, and 2.0 mg of gold chloride was dissolved in 1 mL of deethylene glycol to obtain component B. Fully mix component A and component B in a 5mL reaction bottle to obtain a uniform mixed system. The above mixed system was dried in a vacuum oven to remove the solvent, rinsed three times with 2 mL of ethyl acetate, and dried with nitrogen to obtain gold tellurium nanoparticles. where (HO-EG 2 -C 6 ) 2 The synthesis process of -Te molecule is as follows:
[0072] D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com