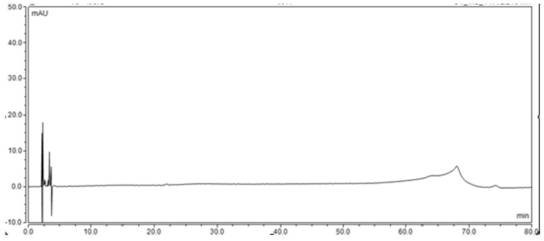
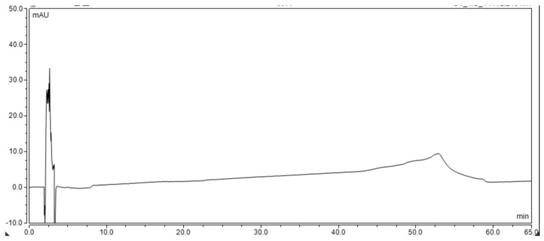
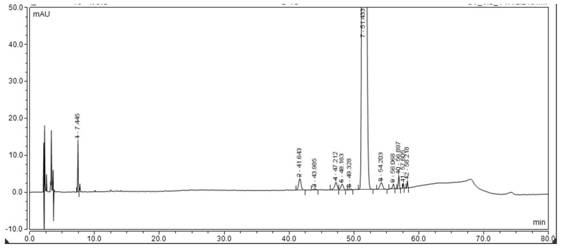
Detection method for related substances in ganirelix acetate injection
A technique for ganirelix and its detection method, which is applied in the field of detection of related substances in ganirelix acetate injection, and can solve problems such as affecting quantitative results, difficulty in accurate integration, poor separation of impurities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Solvent Take 2.35g of mannitol, add 40ml of water to dissolve, add 10mg of glacial acetic acid, mix well, adjust the pH value to 4.9-5.1 with 1mol / L sodium hydroxide solution or 5% (v / v) acetic acid solution, and add water to 50ml.
[0036] Impurity stock solution Accurately weigh 5 mg of the above-mentioned known impurity reference substance into a 10 ml volumetric flask, dissolve with a solvent and dilute to a constant volume to make a solution containing about 0.5 mg / ml as the impurity stock solution.
[0037] System Suitability Solution Precisely weigh 12.5 mg of ganirelix acetate reference substance into a 25 ml volumetric flask, pipette 125 μl of each impurity stock solution into it, dilute with solvent to make ganirelix acetate containing about 0.5 mg, A solution of 2.5 μg of each impurity was used as a system suitability solution.
[0038] Chromatographic conditions:
[0039] Use octadecylsilane bonded silica gel as a filler (WELCH Ultimate LP-18 4.6×250mm, 3μm...
Embodiment 2
[0047] Solvent, impurity stock solution and system suitability solution are the same as in Example 1.
[0048] API stock solution:
[0049] Precisely weigh 5 mg of ganirelix acetate API (API) into a 10 ml volumetric flask, dissolve it with a solvent and dilute to a constant volume to make a solution containing about 0.5 mg / ml as the API stock solution.
[0050] Mixed impurity solution:
[0051] Precisely pipette 20 μl each of the impurity 5 and impurity 7 stock solutions, 60 μl each of the impurity 3 and impurity 6 impurity stock solutions, and 40 μl each of the remaining impurities and the API stock solution into a 100ml volumetric flask, dissolve and dilute to volume with a solvent, and shake well. .
[0052] Chromatographic conditions:
[0053] Use octadecylsilane bonded silica gel as a filler (WELCH Ultimate LP-18 4.6×250mm, 3μm or a chromatographic column with equivalent performance);
[0054] Detection wavelength 210nm;
[0055] Column temperature 50°C;
[0056] Mobi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com