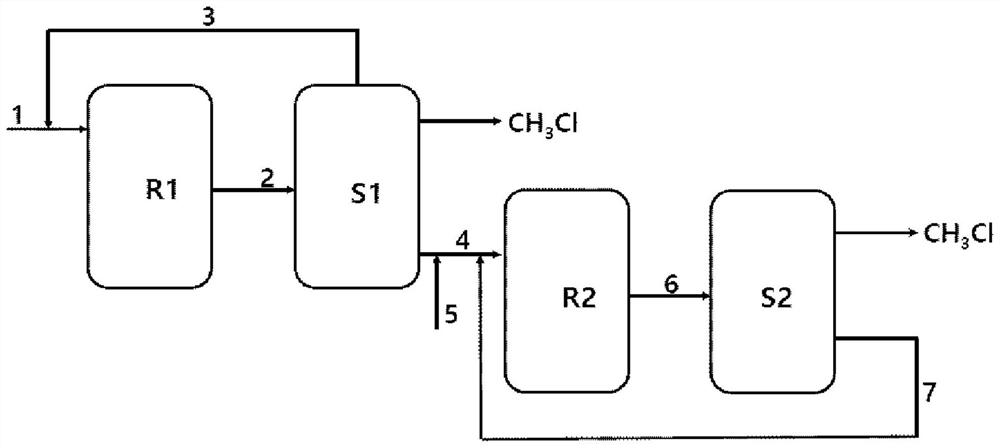
Method of producing methyl chloride by multistage reactions
A manufacturing method and technology of methyl chloride, which are applied in chemical instruments and methods, preparation of halogenated hydrocarbon disproportionation, chemical recovery, etc., can solve the problems of ineffective utilization of hydrogen chloride and unsatisfactory production of methyl chloride.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0079] Next, the present invention will be described in more detail based on the following examples.
[0080] However, the following examples are merely examples of the present invention, and the content of the present invention is not limited to the following examples.
manufacture example 1
[0081] Production of Sulfated Zirconia Catalyst
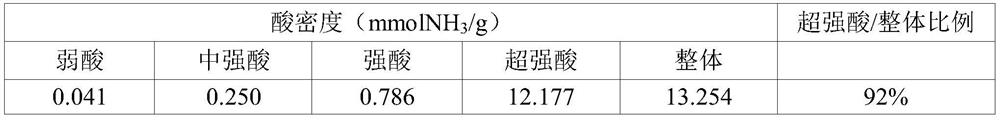
[0082] 2g of glycine (glycine) (Aldrich) and 12.32g of ZrO(NO 3 ) 2 .xH 2 After O(Aldrich) was mixed at a molar ratio of 1:2, the mixture was dissolved in deionized water at a ratio of 2 ml per gram to form an aqueous solution. The aqueous solution formed above was stirred at 80° C. for 2 hours to form a highly viscous gel-like product. The above gel-like product was put into an electric furnace and calcined at 350° C. for 3 hours to form zirconia powder (ZrO 2 powder). The zirconia powder formed above was immersed in a chlorosulfonic acid / dichloroethane solution at a rate of 15 ml per gram, and then heated at 120° C. for 24 hours to evaporate the solvent. After evaporating the solvent, the remaining product was placed in an electric furnace and fired at 650° C. for 3 hours in an air atmosphere to produce 3.6 g of the final product. In the final product produced above, sulfate ion (SO 4 2- ) content was 32.2% by weigh...
manufacture example 2
[0087] Production of Sulfated Tin Oxide Catalyst
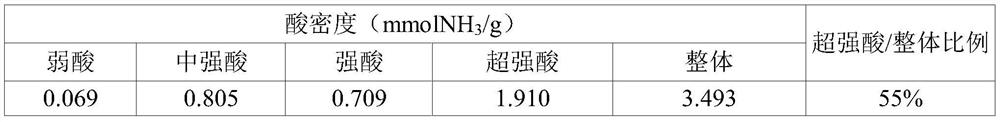
[0088] 25g of SnCl 4 .5H 2 After O is dissolved in 500ml of distilled water, add ammonia water (28%) while stirring at room temperature until the pH of the aqueous solution reaches 8, inducing SnCl 4 .5H 2 O hydrolysis reaction. At this time, the precipitate (precipitate) produced by the hydrolysis reaction was filtered to obtain a solid product, which was fully washed with distilled water, put into an oven, and dried at 110° C. for more than 12 hours, thereby generating 5.5 g of tin hydroxide (Sn (OH) 4 ). The resulting tin hydroxide (Sn(OH) 4 ) in a ratio of 15ml per gram into an aqueous sulfuric acid solution (concentration of sulfuric acid: 0.5M), stirred for 1 hour, filtered to obtain a solid product, washed with distilled water, put into a drying oven, and Dry at 110°C for 2 hours. The dried solid product was placed in an electric furnace and calcined at 500° C. for 3 hours in an air atmosphere to produce 4.8 g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com