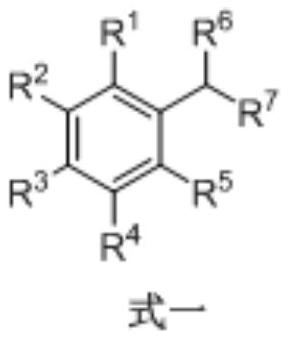
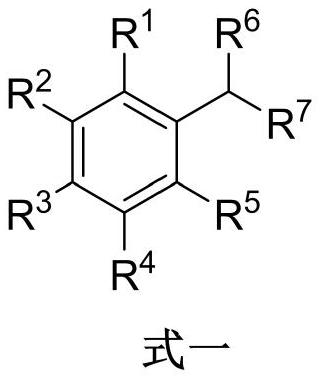
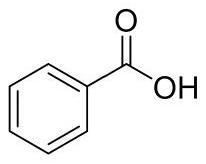
Method for synthesizing acetophenone or benzoic acid compound by oxidizing toluene compound
A technology for benzoic acid and oxidation synthesis, which is applied in the field of synthesizing acetophenone or benzoic acid compounds, can solve the problems of low single conversion rate and limited functional group tolerance, and achieve low cost, mild reaction conditions, and substrate The effect of readily available sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A method for synthesizing benzoic acid by oxidation of toluene, the steps are as follows:
[0021] In a 25mL quartz tube, add toluene 9.2mg, cerium acetate 3.0mg, ethanol 4.0mg, CH 3 CN 2mL. The resulting mixture was irradiated for 12 hours at 80° C. under a 450 nm light source. Remove CH under reduced pressure 3 CN, column chromatography obtained 11.9 mg of the target product, benzoic acid, with a yield of 98%.
[0022] The product structure is
[0023]
[0024] The NMR data of the product are: 1 H NMR (400MHz, DMSO) δ12.98(s, 1H), 7.95(d, J=8.0Hz, 2H), 7.63(t, J=7.2Hz, 1H), 7.50(t, J=7.6Hz, 2H ). 13 C NMR (100 MHz, DMSO) δ 167.4, 132.9, 130.8, 129.3, 128.6.
Embodiment 2
[0026] A method for synthesizing benzoic acid by oxidation of toluene, the steps are as follows:
[0027] In a 25mL quartz tube, add toluene 9.2mg, cerium acetate 3.0mg, ethanol 4.0mg, CH 3 CN 2mL. The resulting mixture was irradiated for 12 hours at 60° C. under a 450 nm light source. Remove CH under reduced pressure 3 CN, column chromatography obtained 10.9 mg of the target product, benzoic acid, with a yield of 90%.
[0028] Product structure and NMR data are the same as in Example 1.
[0029] Compared with Example 1, the difference of this example is that the reaction temperature is 60°C.
Embodiment 3
[0031] A method for synthesizing benzoic acid by oxidation of toluene, the steps are as follows:
[0032] In a 25mL quartz tube, add toluene 9.2mg, cerium acetate 3.0mg, ethanol 4.0mg, CH 3 CN 2mL. The resulting mixture was irradiated for 12 hours at 80°C under a 400nm light source. Remove CH under reduced pressure 3 CN, column chromatography obtained 11.2 mg of the target product, benzoic acid, with a yield of 92%.
[0033] Product structure and NMR data are the same as in Example 1.
[0034] Compared with Embodiment 1, the difference of this embodiment is that a 400nm light source is used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com