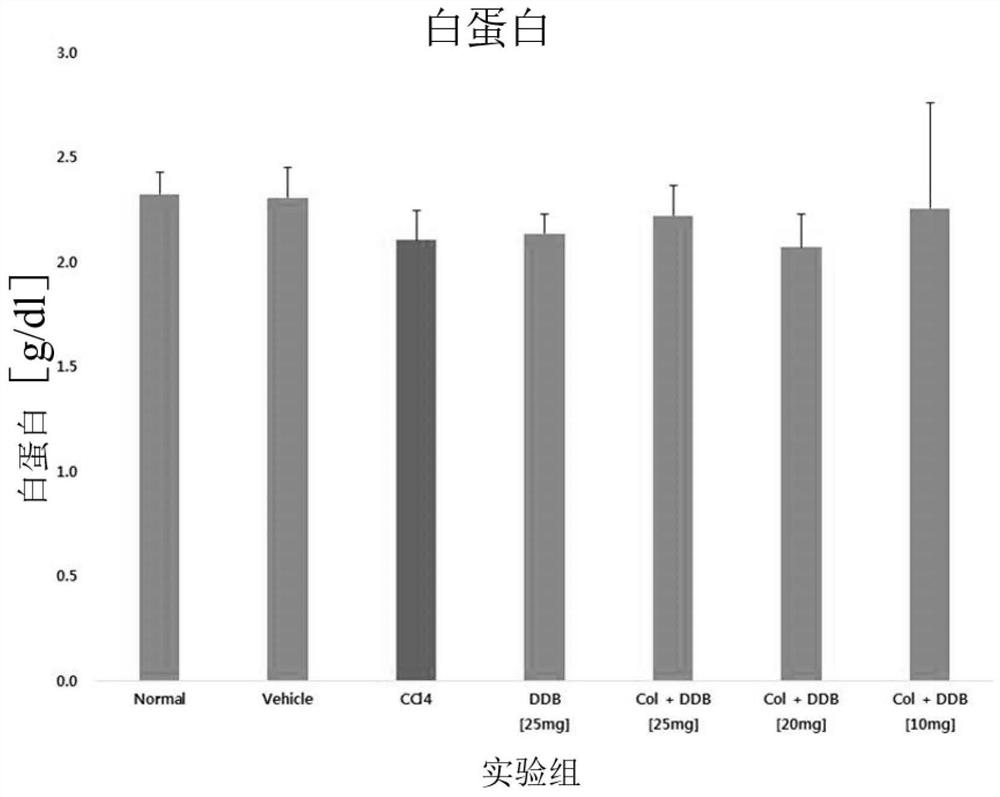
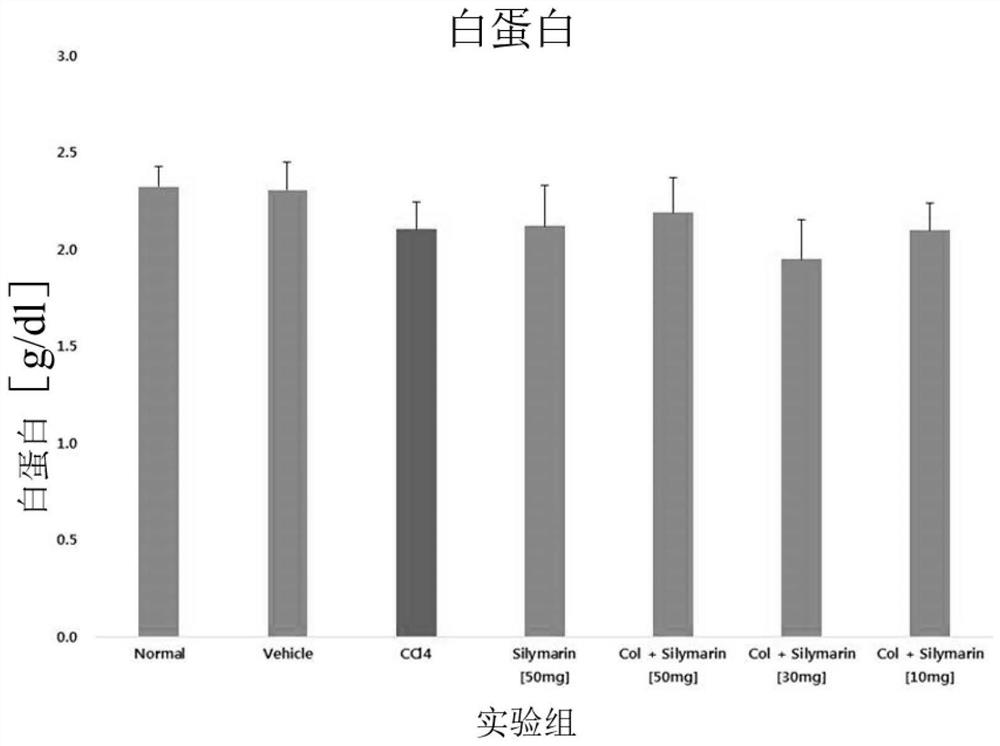
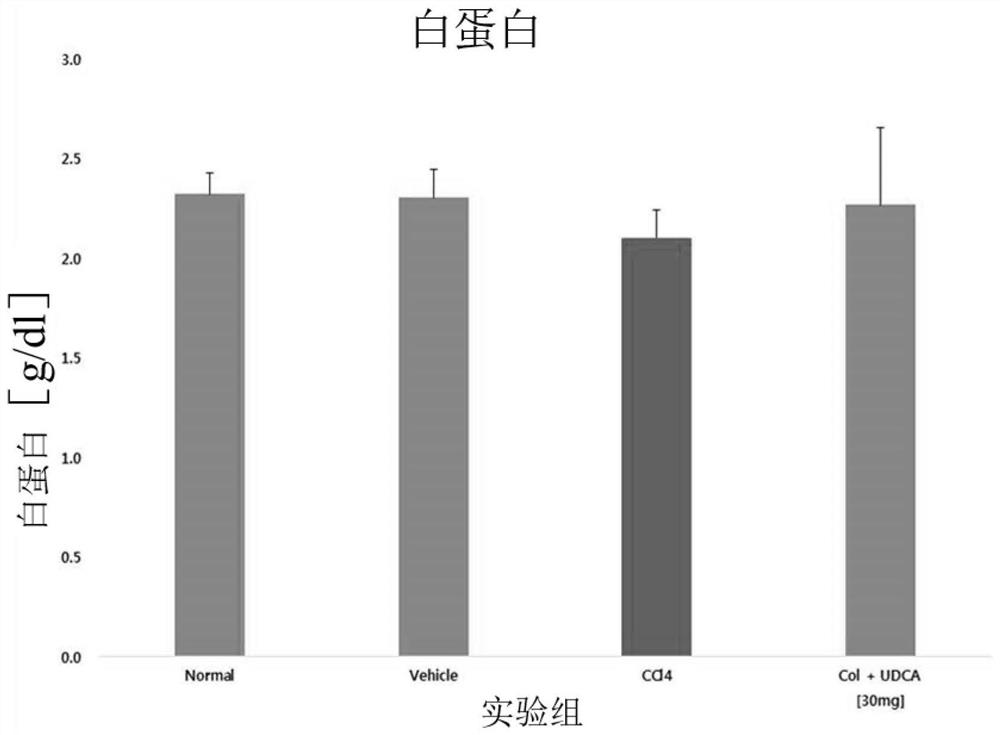
Combination formulation containing colchicine for treatment or enhancing the therapy of liver disease
A technology for colchicine and liver disease, applied in the field of compound preparations, can solve problems such as no research proof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1. Experimental preparation and experimental method
[0078] 1-1. Preparation of experimental animals
[0079] Male Wistar rats [about 150g, 4-5 weeks old].
[0080] 1-2. Drugs and dosing schedule
[0081] The administration plan of carbon tetrachloride (CCl4) and colchicine (Colchicine) used in this experiment is as follows:
[0082] 1. Carbon tetrachloride: intraperitoneal administration of 0.4g / kg bw, 3 times a week, continuous administration for 10 weeks.
[0083] 2. Colchicine: 10, 50, 100 μg / kg bw orally, once a day, for 10 weeks.
[0084] 1-3. Classification of experimental groups
[0085] For the purpose of this experiment, the groups were classified as follows, with 12 animals in each group.
[0086] 1. Control group (distilled water administration group).
[0087]2. Vehicle administration group (only CCl4 solvent olive oil administration group).
[0088] 3. CCl4 administration group [0.4g / kg].
[0089] 4. CCl4 + colchicine [10 μg / kg].
[...
Embodiment 2
[0095] Example 2. Confirm the improvement of the curative effect of colchicine (Colchicine) on liver fibrosis under different dosages
[0096] 2-1. Quantification of hydroxyproline detection
[0097] Hydroxyproline was detected in each group to confirm the cumulative value of cellulose in the liver.
[0098] 【Table 1】
[0099] test group Hydroxyproline [μg / g liver] Normal 274.81±17.49 Vehicle 247.86±23.10 CCl4[0.4g / kg] 336.36±18.82 CCl4+ colchicine [10μg / kg] 320.12±13.32 CCl4+ colchicine [50μg / kg] 313.33±12.50 CCl4+ colchicine [100μg / kg] 302.25±10.25
[0100] As shown in Table 1, CC14-induced hepatic fibrosis exhibited a cellulose-reducing effect in the colchicine-administered group.
Embodiment 3
[0101] Embodiment 3. Experimental preparation and experimental method
[0102] 3-1. Preparation of experimental animals
[0103] Male Wistar rats [about 150g, 4-5 weeks old].
[0104] 3-2. Drugs and dosing schedule
[0105] The dosing schedule of carbon tetrachloride (CCl4), silymarin (Silymarin), colchicine (Colchicine) and dimethyl biphenyl dicarboxylate (DDB) used in this experiment is as follows:
[0106] 1. Carbon tetrachloride: intraperitoneal administration of 0.4g / kg·bw, 3 times a week, continuous administration for 10 weeks.
[0107] 2. Silymarin: Oral administration of 10, 30, 50 mg / kg·bw, once a day, for 10 consecutive weeks.
[0108] 3. Colchicine: Oral administration of 10 μg / kg bw, once a day, for 10 consecutive weeks.
[0109] 4. Dimethyl diphenylcarboxylate (DDB): Oral administration of 10, 20, 25 mg / kg·bw, once a day, for 10 consecutive weeks.
[0110] 5. UDCA: Oral administration of 30mg / kg·bw, once a day, continuous administration for 10 weeks.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com