Four-membered ring-containing hetero-diterpenoid compound, preparation method and application thereof, and pharmaceutical composition
A technology of compounds and diterpenoids, applied in the preparation of organic compounds, drug combinations, compounds of Group 4/14 elements of the periodic table, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The embodiment of the present invention also provides a preparation method of the above-mentioned four-membered ring-containing heteroditerpenoid compound, which includes:
[0045] Compound I is oxidized and then deprotected to obtain heteroditerpene compound A;
[0046] The heteroditerpene compound A is esterified or etherified to obtain the heteroditerpene compound B; wherein, the structural formula of the compound I is
[0047] In the formula, PG is a hydroxyl protecting group selected from TBDPS, TMS, TES, TBDMS or TIPS.
[0048] The oxidation of compound I is achieved by an oxidizing agent, for example, sodium chlorite can be used as an oxidizing agent. The oxidizing agent can oxidize the aldehyde group in the compound I to a carboxyl group, and then remove the protecting group on the hydroxyl group to obtain the heteroditerpene compound A. The quality of the oxidant is 0.1-1 times that of the compound I, and the oxidation of the aldehyde group is relatively su...
Embodiment 1
[0069] This embodiment provides a kind of preparation method of compound IV-a, and its reaction formula is as follows:
[0070]
[0071] Its specific preparation steps include:
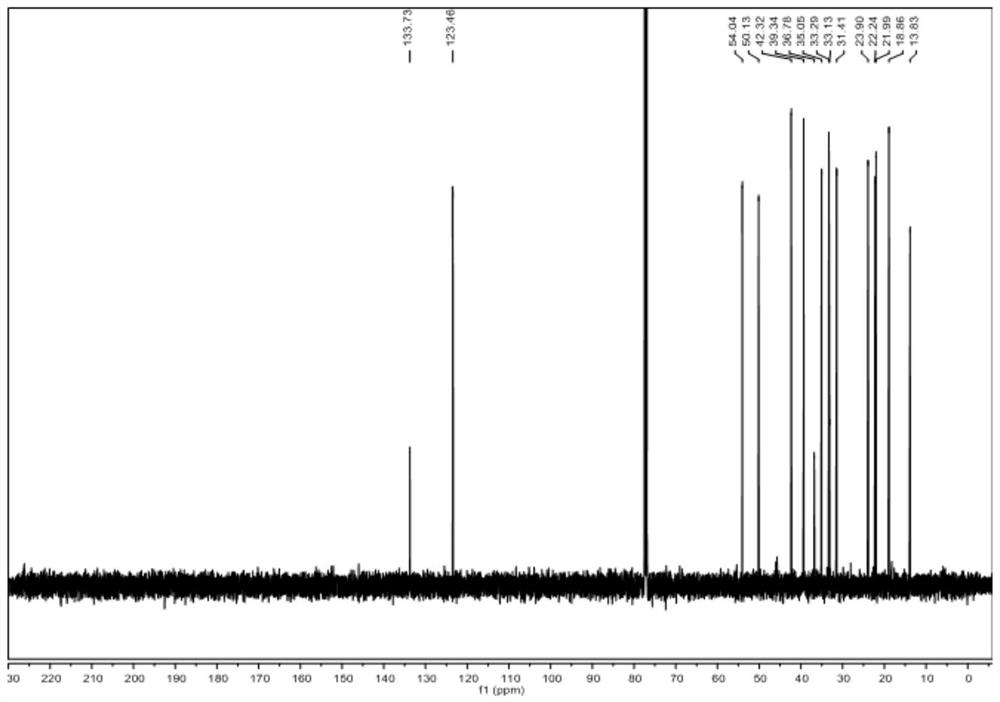
[0072] Dissolve raw material V-a (0.01~100g) and raw material VI-a in (1~100mL) 1,2-dichloroethane or dichloromethane, add (0.01~10mL) organic base, at -40~25℃ Add Tf dropwise 2 O (0.01-20mL) in 1,2-dichloroethane or dichloromethane solution, react at -20-25°C for 1-48 hours, spot plate to detect the disappearance of raw materials, add inorganic alkali aqueous solution or water 1-200mL, Stir at room temperature or under heating for 1 to 10 hours. The new product is no longer increased by spot plate detection. Extract with an equal volume of organic solvent for 2 to 6 times, combine the filtrate, concentrate the sample on a rotary evaporator, and obtain a white solid by various purification methods. It was identified as compound IV-a by mass spectrometry, one-dimensional and two-dimensional nuclea...
Embodiment 2
[0077] This embodiment provides a kind of preparation method of compound IV-a, and its reaction formula is as follows:
[0078]
[0079] Its specific preparation steps include:
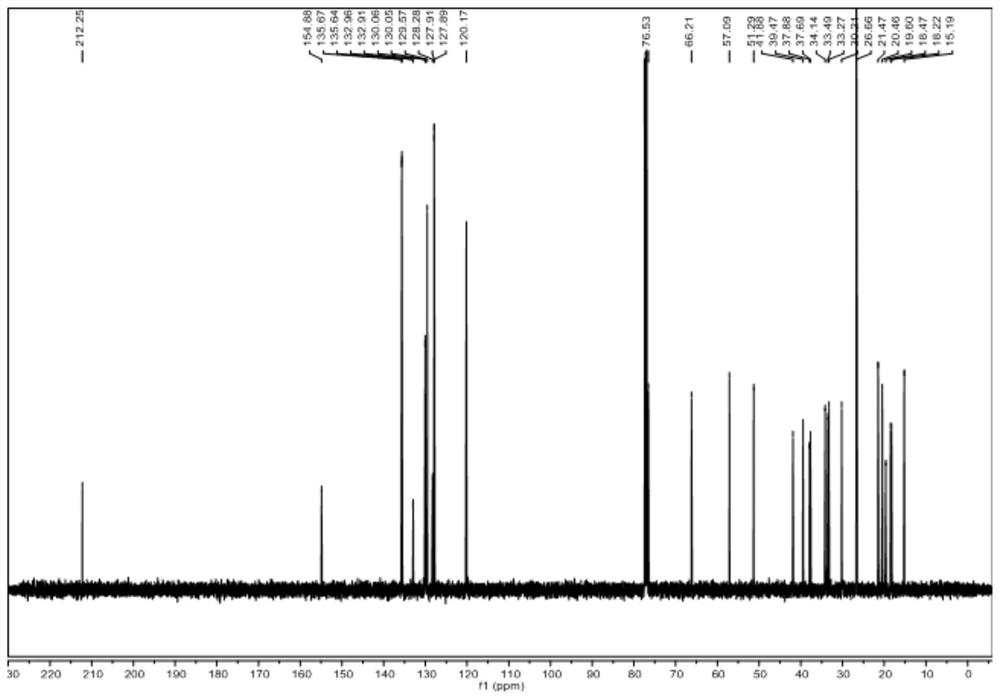
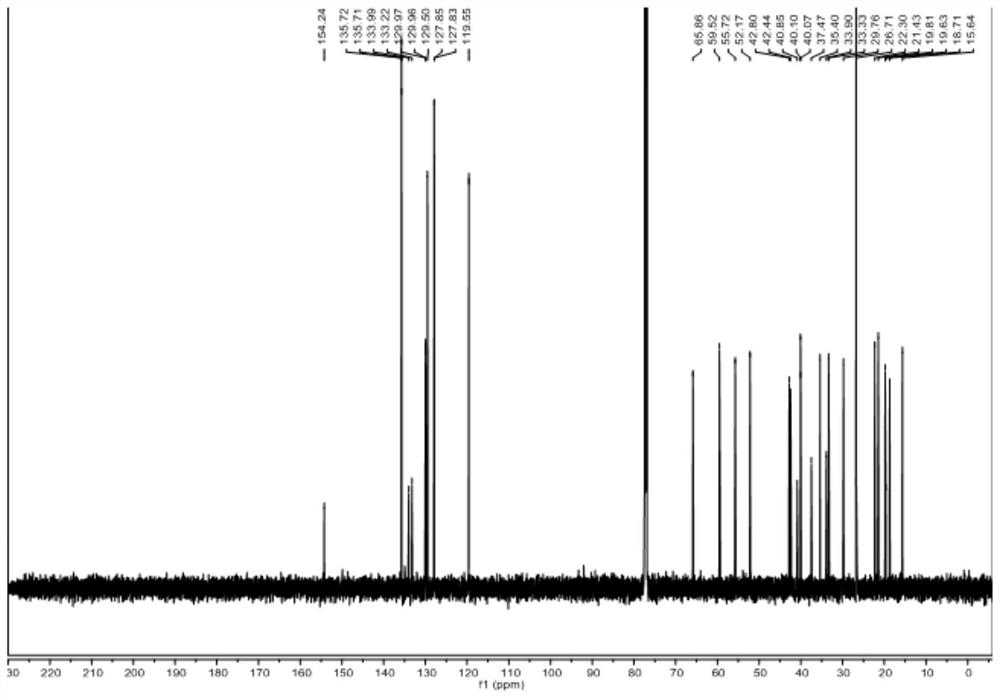
[0080] Dissolve raw material V-b (0.01~100g) and raw material VI-b in (1~100mL) 1,2-dichloroethane or dichloromethane, add (0.01~10mL) organic base, at -40~25℃ Add Tf dropwise 2 O (0.01-20mL) in 1,2-dichloroethane or dichloromethane solution, react at -20-25°C for 1-48 hours, spot plate to detect the disappearance of raw materials, add inorganic alkali aqueous solution or water 1-200mL, Stir at room temperature or under heating for 1 to 10 hours. The new product is no longer increased by spot plate detection. Extract with an equal volume of organic solvent for 2 to 6 times, combine the filtrate, concentrate the sample on a rotary evaporator, and obtain a white solid by various purification methods. It was identified as compound IV-b by mass spectrometry, one-dimensional and two-dimensional nuclea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com