Prediction method for unit mass combustion heat of hydrocarbon fuel
A unit mass, hydrocarbon fuel technology, applied in special data processing applications, design optimization/simulation, etc., can solve problems such as large errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
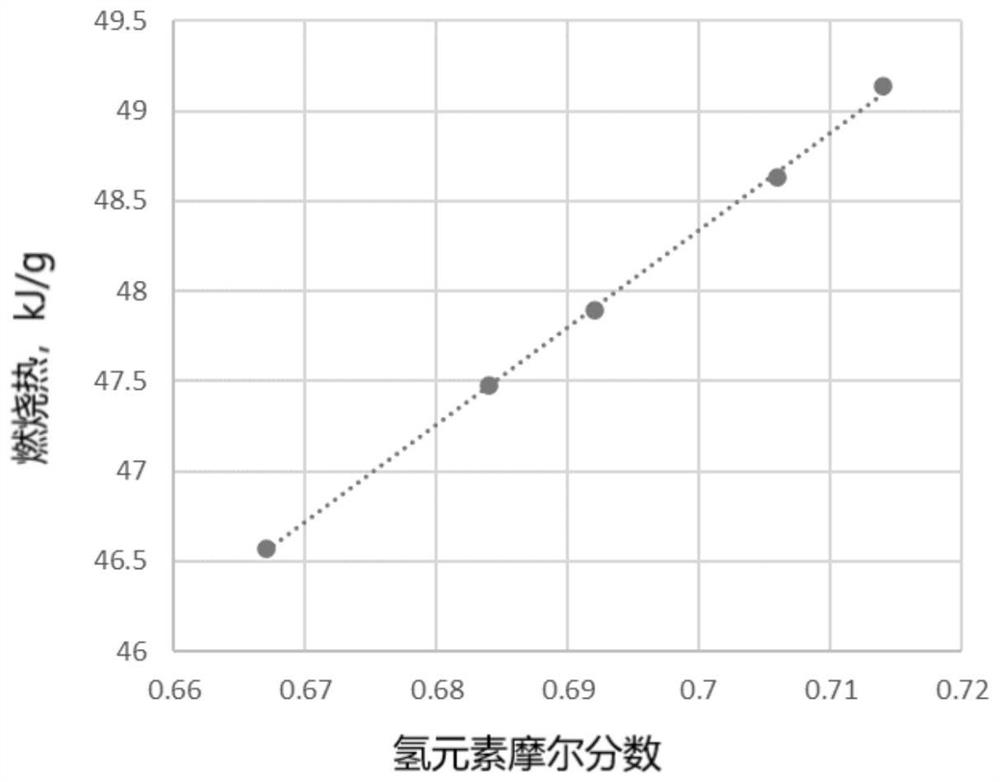
[0063] Prediction of the gaseous heat of combustion of 2-methylheptane. The molecular formula for 2-methylheptane is C 8 h 18 , the hydrogen mole fraction of 2-methylheptane is:
[0064]
[0065] In the 2001 edition of "Practical Chemistry Handbook" published by Science Press, the gaseous heat of combustion per unit mass of methane, ethane, propane, butane, pentane, octane, dodecane and cyclohexane was found, and the unit mass of combustion The heat is divided by the molecular weight to convert the heat of combustion per unit mass, and at the same time, the hydrogen mole fraction of the selected compound is calculated according to the step of calculating the hydrogen mole fraction of 2-methylheptane, and the data are listed in Table 1.
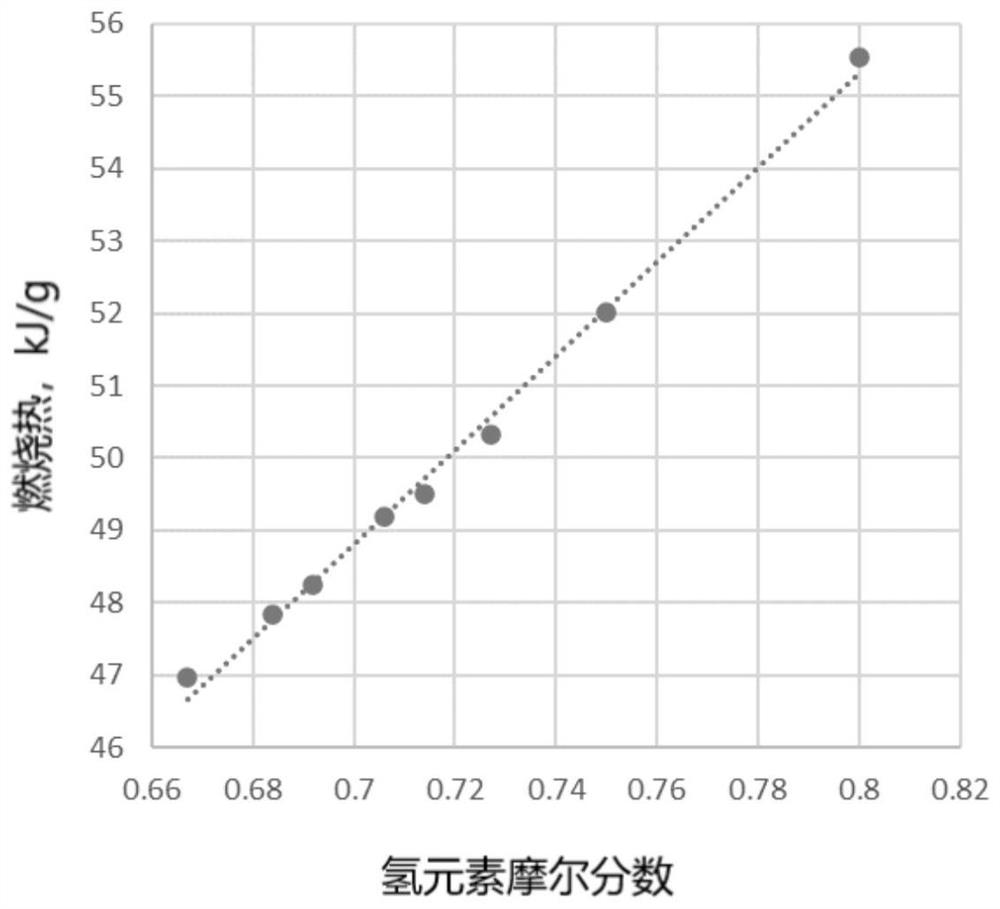
[0066] Draw the relationship between the gaseous heat of combustion of saturated hydrocarbon fuel and the mole fraction of hydrogen (attached figure 1 ), and fit the gaseous combustion heat prediction mathematical expression:
[0067] G...
Embodiment 2
[0076] Prediction of high calorific value of natural gas in Zhongwu line. From the "Domestic Natural Gas Composition Table" on the Daoke Baba website, it is found that the methane content of the Zhongwu Line natural gas is 97.00%, the ethane content is 1.5%, the propane content is 0.5%, and the nitrogen content is 1%. Methane, ethane, propane, and nitrogen have molecular weights of 16, 30, 44, and 28, respectively. The number of carbon atoms per molecule of methane, ethane and propane are 1, 2 and 3 respectively; the number of hydrogen atoms per molecule of methane, ethane and propane are 4, 6 and 8 respectively.
[0077] The equivalent molecular weight of natural gas=97.00%×16+1.5%×30+0.5%×44+1%×28=16.19
[0078] Relative molar content of carbon in natural gas=97.00%×1 / 16+1.5%×2 / 30+0.5%×3 / 44
[0079] =0.0619
[0080] Relative molar content of hydrogen in natural gas=97.00%×4 / 16+1.5%×6 / 30+0.5%×8 / 44
[0081] =0.2464
[0082] The relative molar content of elements in the Zh...
Embodiment 3
[0089] Prediction of the heat of combustion in the liquid state of tert-butylcyclohexane. The molecular formula for tert-butylcyclohexane is C 10 h 20 , the hydrogen mole fraction of tert-butylcyclohexane is:
[0090]
[0091] The heat of combustion in the liquid state of butane, pentane, octane, dodecane, and cyclohexane was found in the 2001 edition of "Practical Chemistry Handbook" published by Science Press, and converted to unit mass after dividing the heat of combustion per unit mass by the molecular weight Heat of combustion. The hydrogen mole fraction of the selected compound was calculated according to the calculation method of the hydrogen mole fraction of tert-butylcyclohexane, and the data are listed in Table 1.
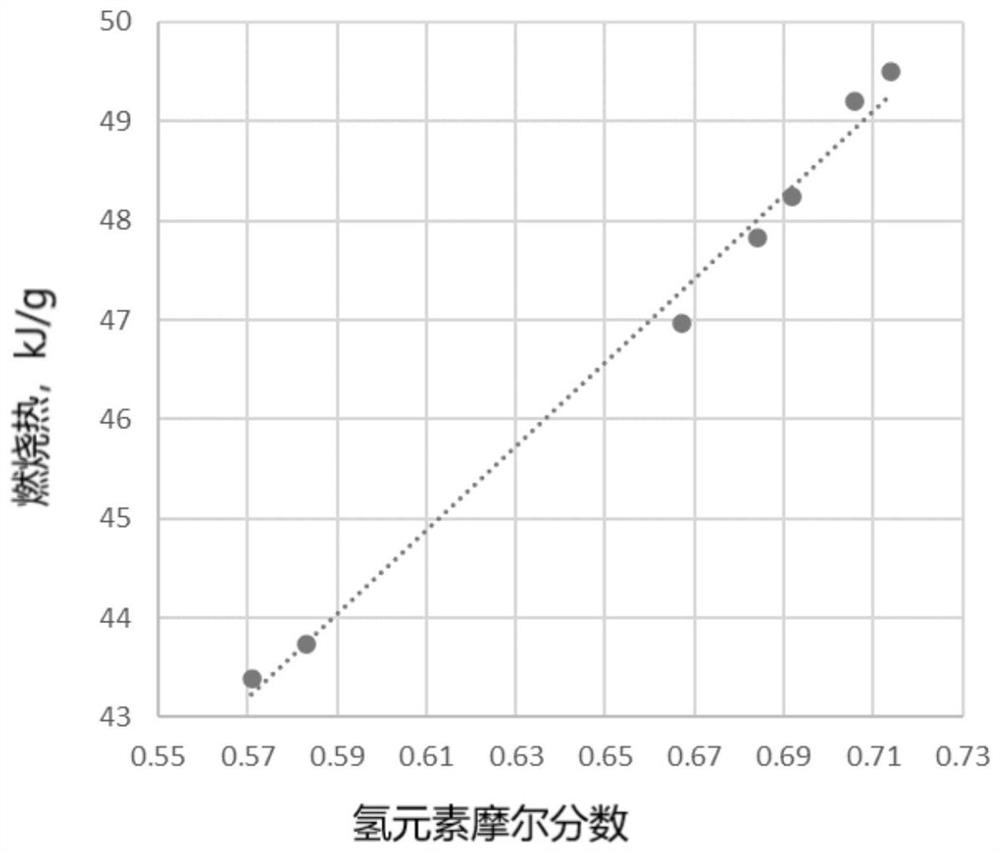
[0092] Draw the relationship between the liquid heat of combustion of saturated hydrocarbon fuel and the mole fraction of hydrogen (attached figure 2 ), and fit the mathematical expression of liquid combustion heat prediction;
[0093] Liquid com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com