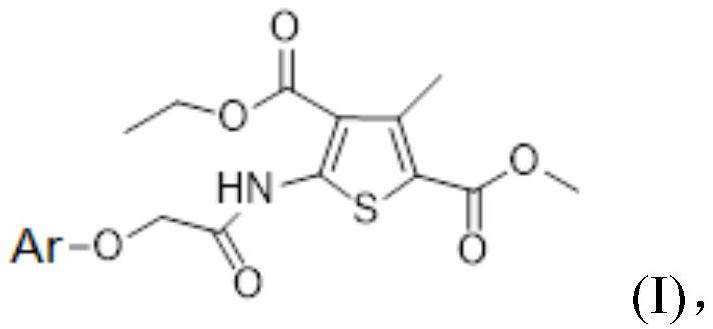
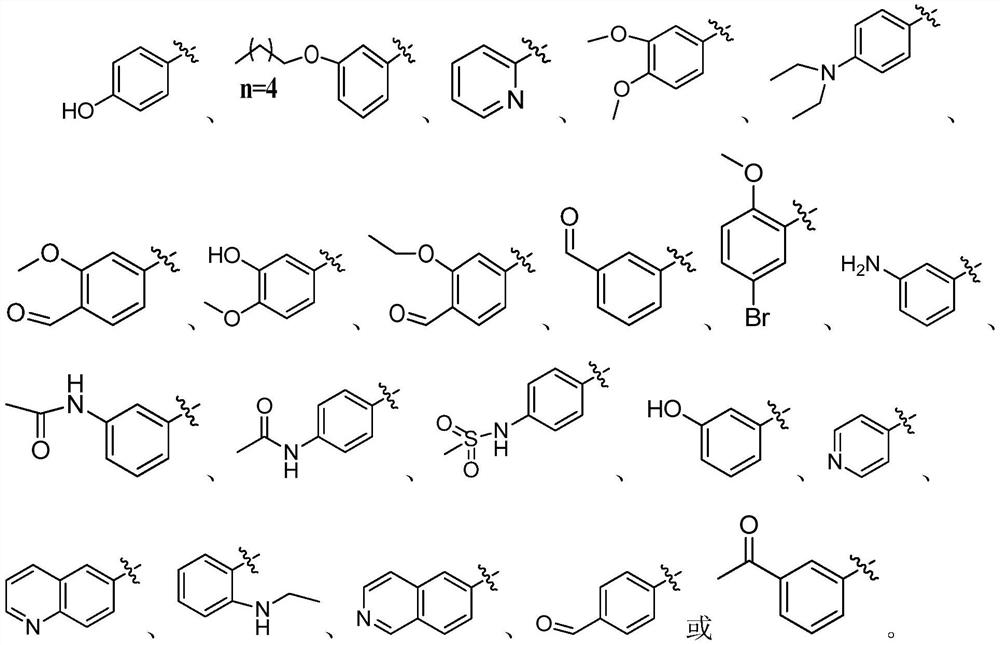
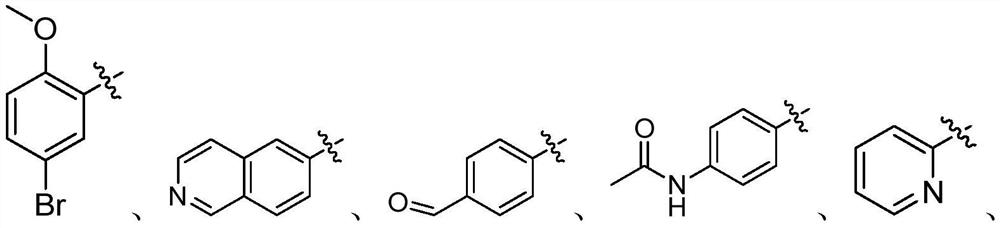
2-aminothiophene neuraminidase inhibitor, preparation method and application thereof
A technology of neuraminidase and aminothiophene, which is applied in the field of biomedicine, can solve the problems of expensive raw materials for Tamiflu production and complicated synthesis process, and achieve excellent inhibitory effect, simple synthesis method, and good neuraminidase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] Described preparation method specifically comprises the following steps:
[0046] (1) methyl acetoacetate, sublimed sulfur and ethyl cyanoacetate form a reaction system, and after the reaction, obtain the intermediate of formula (II);
[0047] (2) The intermediate of formula (II) obtained in step (1) is dissolved in an organic solvent and forms a reaction system with chloroacetyl chloride, and the intermediate of formula (III) is obtained after the reaction;
[0048] (3) The intermediate of formula (III) obtained in step (2) and the substituted aromatic phenol are dissolved in an organic solvent to form a reaction system, and after the reaction, the 2-aminothiophene inhibitor shown in formula (I) is obtained through aftertreatment;
[0049] In step (1), diethylamine is used as the catalyst, absolute ethanol is used as the organic solvent, the temperature of the reaction system is 25-80°C, preferably 25°C, and the reaction time is 14-48h, preferably 26h, the post-treatm...
Embodiment 1
[0079] 4-Ethyl-2-methyl-5-(2-(5-bromo-2-methylphenoxy)acetamido)-3-methylthiophene-2,4-dicarboxylate, its structural formula is as follows Shown in formula I:
[0080]
[0081] Concrete synthetic steps are as follows:
[0082] (1) Accurately measure 10.78mL (100mmoL) methyl acetoacetate and 10.65mL (100mmoL) ethyl cyanoacetate with a graduated cylinder, pour them into a 50ml round bottom flask, and then accurately weigh 3.21g (100mmoL) of sublimed sulfur into the round Bottom flask, pour 20mL absolute ethanol, stir. Add 8 mL of diethylamine dropwise with a constant pressure dropping funnel. After the dropwise addition, the constant-pressure funnel was removed, and placed at room temperature at 25° C. for a stirring reaction for 26 hours. After the reaction, a large amount of solids were produced in the system solution. The solid was filtered to obtain a filter cake, which was washed several times with 50% ethanol aqueous solution, and then dried at normal temperature to ...
Embodiment 2
[0089] 4-Ethyl-2-methyl-5-(2-(quinoline-6-acyloxy)-acetamido)-thiophene-2,4-dicarboxylate, its structural formula is as follows, similar to Example 1 prepared by the method.
[0090]
[0091] White solid, 94% yield, IC 50 The value is 0.035 μM.
[0092] 1 H NMR (500MHz, CDCl 3)δ12.58(s,1H),8.87–8.82(m,1H),8.11(t,J=9.5Hz,2H),7.63(dd,J=9.5,3.0Hz,1H),7.42(dd,J =8.0,4.0Hz,1H),7.17(d,J=2.5Hz,1H),4.89(s,2H),4.45(q,J=7.0Hz,2H),3.88(s,3H),2.79(s ,3H),1.44(t,J=7.0Hz,3H). 13 C NMR (125MHz, CDCl 3 )δ165.99,165.85,163.21,154.97,151.00,148.85,144.97,135.07,131.62,128.98,122.02,121.70,120.36,117.76,115.34,106.97,615.12,614.29,
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com