Method for constructing On-DNA alpha-amino amide compound through aqueous-phase Ugi multi-component reaction
A multi-component reaction and aminoamide technology, applied in the field of coding compound libraries, can solve problems such as low efficiency and inconvenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
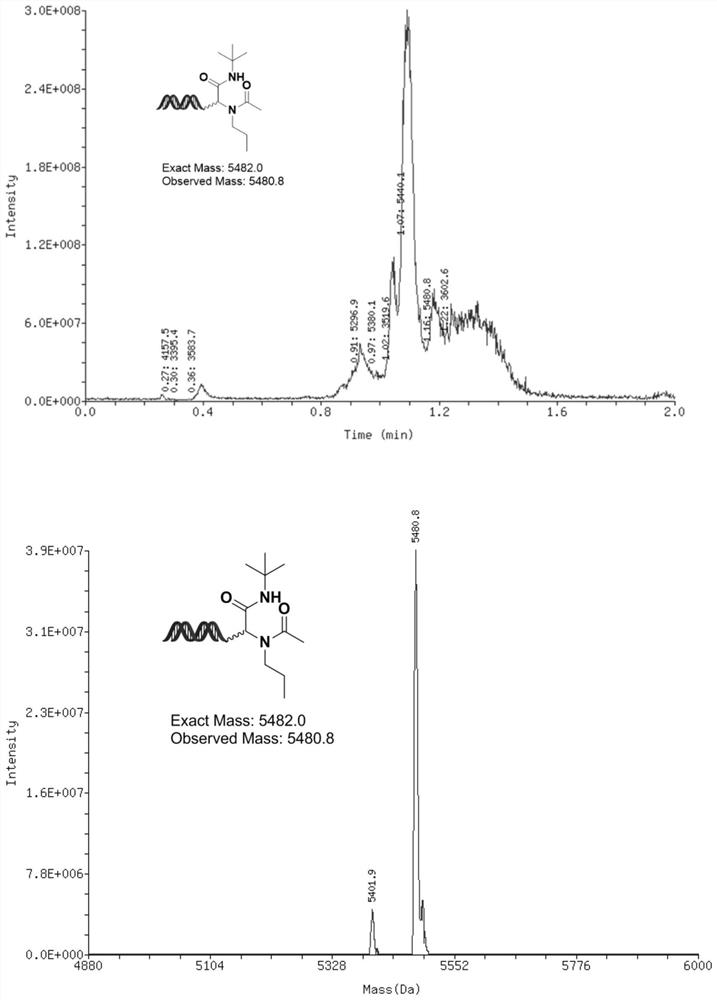
[0142] The synthesis of embodiment 1, compound 1
[0143]
[0144] Propylamine (6.67μL, 333 molar equivalents, 1M MeOH solution) and DNA-CHO (20μL, 20nmol, 1mM aqueous solution) were pre-mixed evenly, and the reaction solution was activated at room temperature for 2 hours. After activation, add Acetic acid (6.67 μL, 333 molar equivalents, 1M MeOH solution), then add tert-butylisonitrile (6.67 μL, 333 molar equivalents, 1M MeOH solution), mix the reaction solution thoroughly, and then place the reaction solution at 60°C The next 16 hours.
[0145] After the reaction is completed: ethanol precipitation, add 4 μL, 5M sodium chloride solution to the solution, and then continue to add 120 μL absolute ethanol, after shaking evenly, freeze the reaction at -20°C for 2 hours, and then centrifuge at high speed After half an hour, the supernatant was discarded, and the remaining precipitate was dissolved in deionized water to obtain compound 1 with a conversion rate of 15%.
Embodiment 2
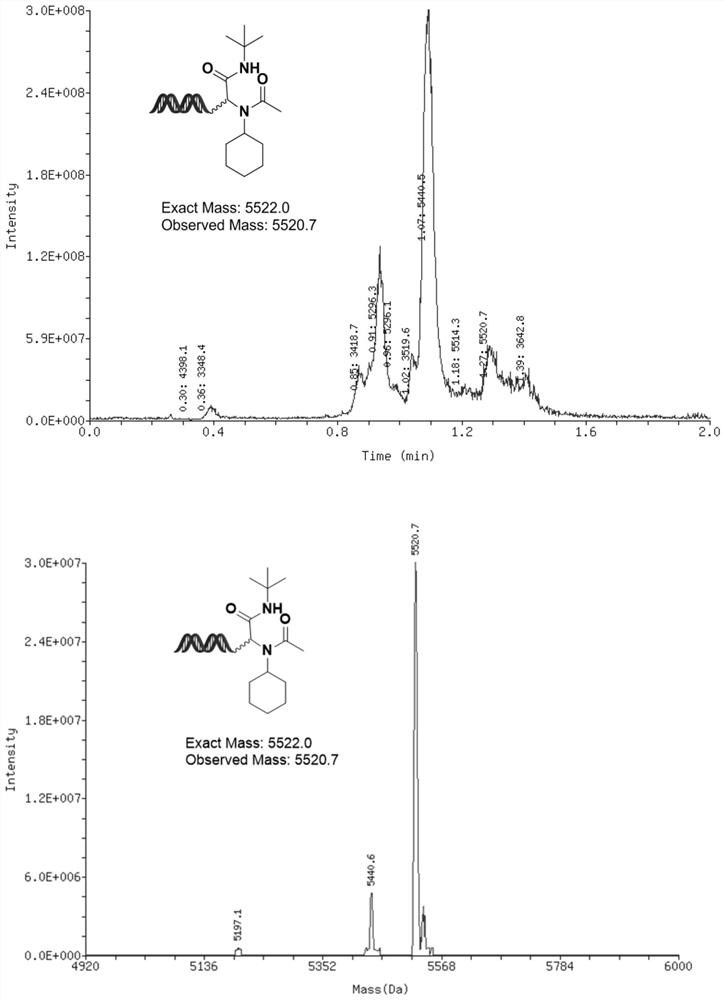
[0146] The synthesis of embodiment 2, compound 2
[0147]
[0148] Cyclohexylamine (6.67 μL, 333 molar equivalents, 1M MeOH solution) and DNA-CHO (20 μL, 20 nmol, 1 mM aqueous solution) were pre-mixed uniformly, and the reaction solution was activated at room temperature for 2 hours. After activation, First add acetic acid (6.67 μL, 333 molar equivalents, 1M MeOH solution), then add tert-butylisonitrile (6.67 μL, 333 molar equivalents, 1M MeOH solution), mix the reaction solution thoroughly, and then place the reaction solution at 60 16 hours under the condition of ℃.
[0149] After the reaction is completed: ethanol precipitation, add 4 μL, 5M sodium chloride solution to the solution, and then continue to add 120 μL absolute ethanol, after shaking evenly, freeze the reaction at -20°C for 2 hours, and then centrifuge at high speed After half an hour, the supernatant was discarded, and the remaining precipitate was dissolved in deionized water to obtain compound 2 with a co...
Embodiment 3
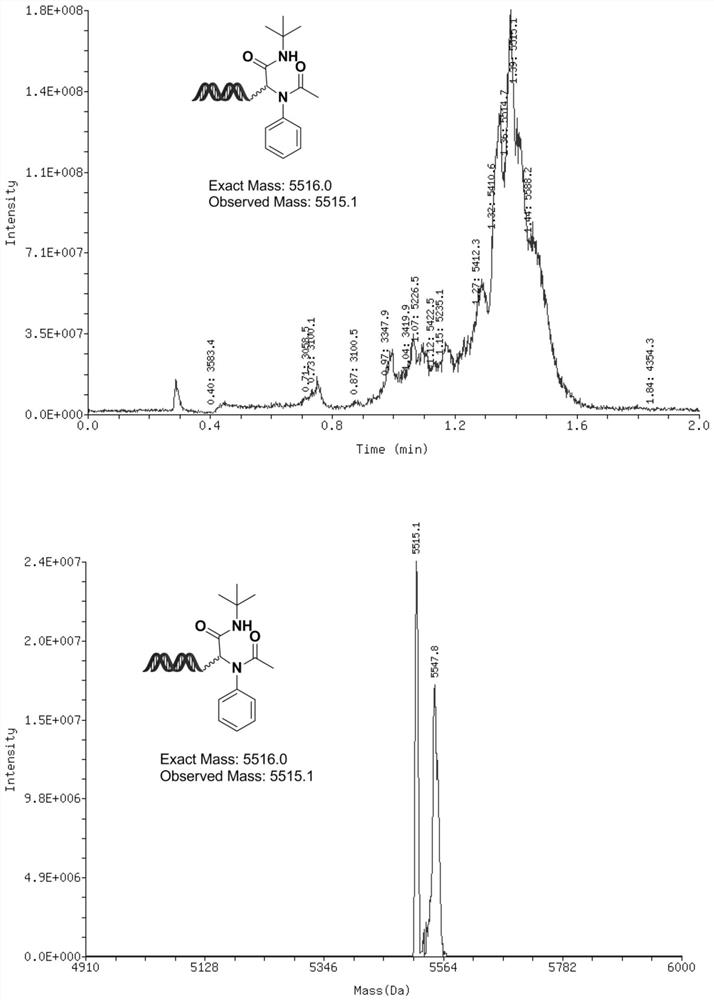
[0150] The synthesis of embodiment 3, compound 3
[0151]
[0152] Mix aniline (6.67μL, 333 molar equivalents, 1M MeOH solution) and DNA-CHO (20μL, 20nmol, 1mM aqueous solution) in advance, and activate the reaction solution at room temperature for 2 hours. After activation, add Acetic acid (6.67 μL, 333 molar equivalents, 1M MeOH solution), then add tert-butylisonitrile (6.67 μL, 333 molar equivalents, 1M MeOH solution), mix the reaction solution thoroughly, and then place the reaction solution at 60°C The next 16 hours.
[0153] After the reaction is completed: ethanol precipitation, add 4 μL, 5M sodium chloride solution to the solution, and then continue to add 120 μL absolute ethanol, after shaking evenly, freeze the reaction at -20°C for 2 hours, and then centrifuge at high speed After half an hour, the supernatant was discarded, and the remaining precipitate was dissolved in deionized water to obtain compound 3 with a conversion rate of 51%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com