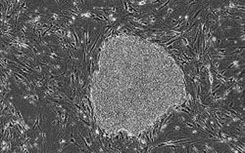
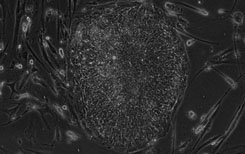
A clinical grade stem cell serum-free medium
A serum-free medium and stem cell technology, which is applied in the field of cell culture medium and clinical-grade stem cell serum-free medium, which can solve the problems of limited availability, high cost, and inability to maintain stem cell stemness well.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] The serum-free medium for clinical-grade stem cells of this embodiment uses DMEM-F12 medium as the basal medium, and removes lipid substances that affect cell differentiation and substances that synthesize lipids in the DMEM-F12 medium: including Inositol and linoleic acid also contain added ingredients. The added ingredients and their content (final concentration) are as follows: polyvinyl alcohol 5ng / ml, BMP-4 10ug / ml, vitamin C 40ug / ml, retinoic acid 1uM, niacinamide 5ug / ml, azithromycin 20ug / ml.
[0011] The specific configuration method is to not add inositol and linoleic acid components when configuring DMEM-F12 medium, or to delipidize DMEM-F12 medium with activated carbon. The above components were added to the DMEM-F12 medium containing no lipid components and synthetic lipid components to achieve the above concentrations, thereby obtaining a serum-free medium for clinical-grade stem cells.
Embodiment 2
[0013] The serum-free medium for clinical-grade stem cells in this example uses E8 medium as the basal medium, and removes lipid components that affect cell differentiation and synthetic lipid components in the E8 medium: including inositol, sub- Oleic acid also contains added ingredients, the added ingredients and their content (final concentration) are as follows: polyvinyl alcohol 10ng / ml, BMP-4 20ug / ml, vitamin C 15ug / ml, retinoic acid 1uM, niacinamide 1ug / ml, azithromycin 30ug / ml.
[0014] The specific configuration method is, when configuring E8 medium, do not add inositol and linoleic acid components, or E8 medium is degreased with activated carbon. The above-mentioned components are added to the E8 medium containing no lipid components and synthetic lipid components, so that the final concentration is the above concentration, thereby obtaining a serum-free medium for clinical-grade stem cells.
Embodiment 3
[0016] Umbilical cord mesenchymal stem cells were cultured in vitro with a clinical-grade stem cell serum-free medium obtained in Example 1, and the steps were as follows:
[0017] (1) Collection and separation of umbilical cord tissue: One umbilical cord from a healthy full-term puerpera was taken. The acquisition process was legal, and the detection items for hepatitis B, hepatitis C, HIV, syphilis, ALT, and mycoplasma were all negative. In the sterile biological safety cabinet of the GMP laboratory, the umbilical cord tissue was washed, disinfected, and the arteriovenous blood vessels and the fetal amniotic membrane tissue were removed. Finally, the perivascular tissue and Wharton's jelly tissue were obtained, which were fully cut into 1mm3 tissue pieces. A clinical-grade stem cell serum-free medium prepared in Example 1 was added by the tissue block adherence method, and seeded into a petri dish or flask at an appropriate density, and cultured at 37°C in a CO2 incubator.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com