Preparation method of deuterated ibrutinib
A technology of ibrutinib and deuteration, which is applied in the field of preparation of deuterated ibrutinib, can solve the problems of poor selectivity of deuterated sites, highly toxic raw materials, low deuterated rate, etc., and achieve good catalytic effect and simple operation , good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
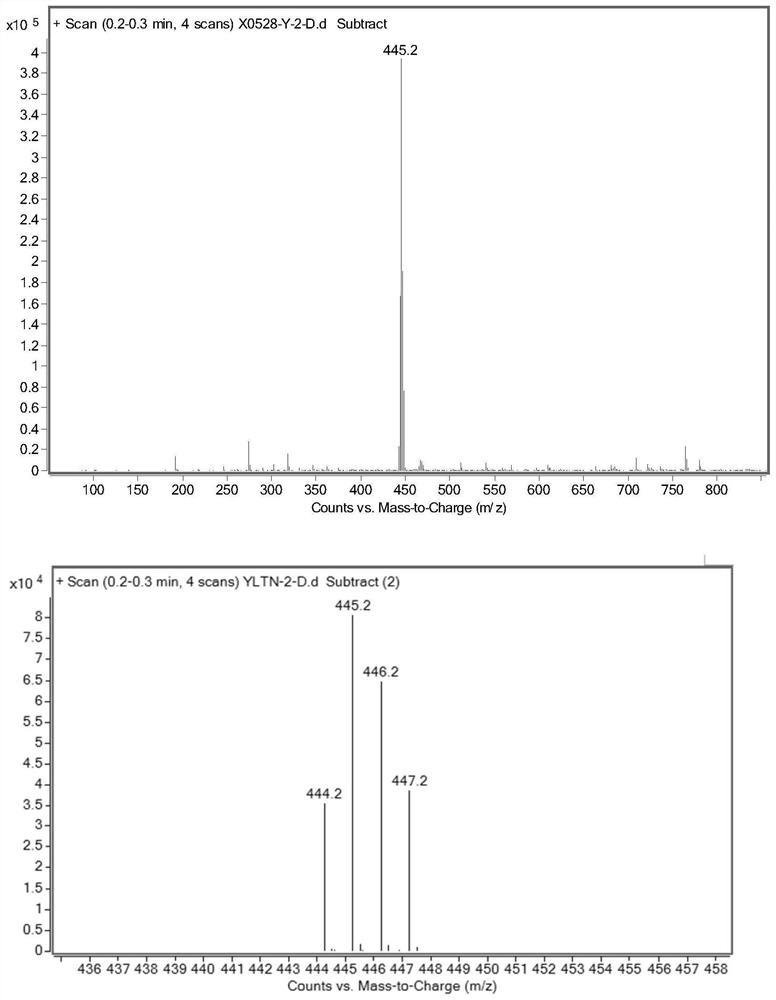
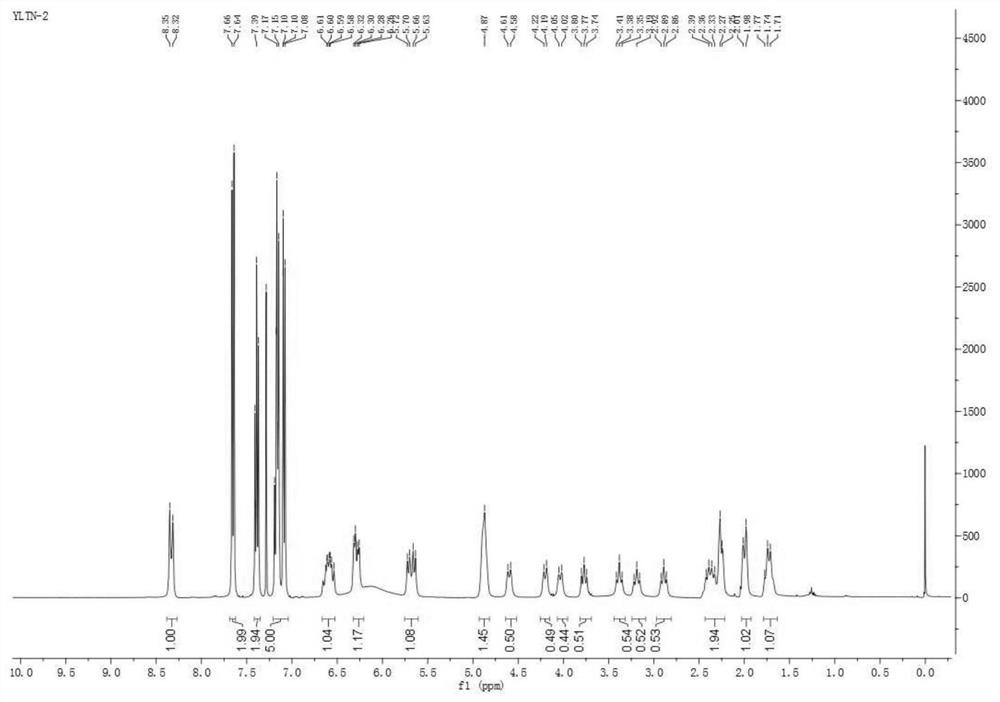
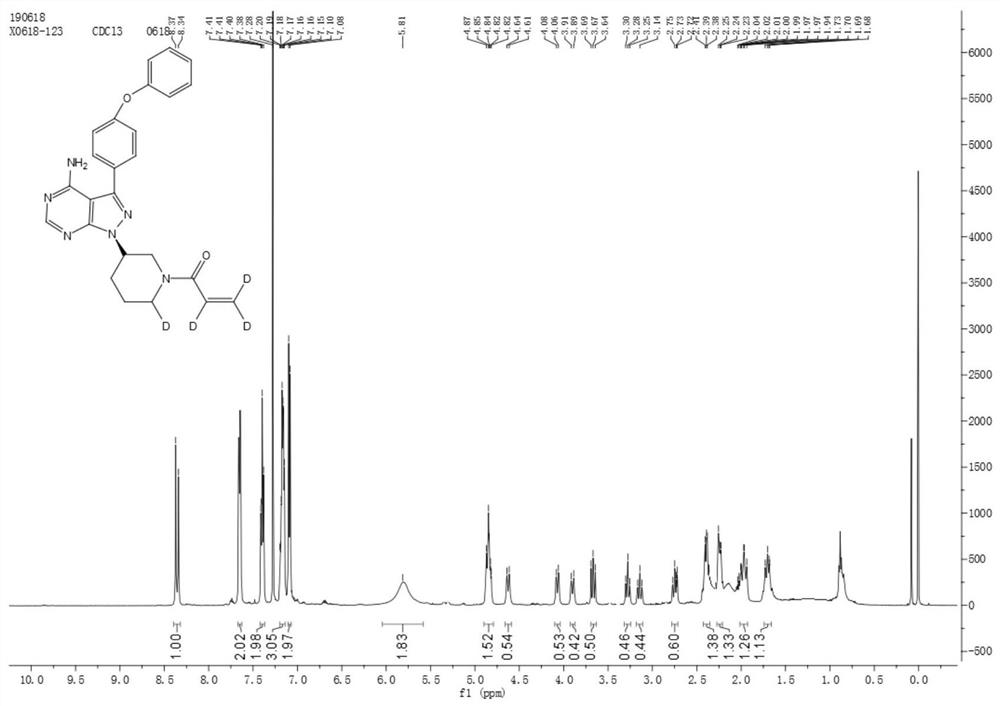
[0034] Under the protection of an inert gas, 4CzIPN (63.04mg, 0.08mmol, 0.02eq), ibrutinib (1.76g, 4.0mmol, 1.0eq), Li 2 CO 3 (355mg, 4.8mmol, 1.2eq), NMP (30mL), triisopropylsilanethiol (228mg, 1.2mmol, 0.3eq) and D 2 O (4 mL, 200 mmol, 50 equiv). The reaction mixture was irradiated with a 25W 395nm blue LED light setting for 24 hours at room temperature. The crude product was purified by flash chromatography to give a white solid (1.2501 g, 70.2% yield).
Embodiment 2
[0036] Under the protection of inert gas, add Cu[(BCP)(Xantphos)]PF in sequence 6 (34.2mg, 0.03mmol, 0.03eq), ibrutinib (0.44g, 1mmol, 1.0eq), Li 2 CO 3 (74mg, 1.0mmol, 1.0eq), NMP (10mL), triphenylsilanethiol (87.7mg, 0.3mmol, 0.3eq) and D 2 O (0.2 mL, 10 mmol, 10 equiv). The reaction mixture was irradiated for 1 hour at 150°C with a 25W 395nm blue LED light setting. The crude product was purified by flash chromatography to give a white solid (0.056 g, 12.73% yield).
Embodiment 3
[0038] Under the protection of inert gas, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (33.6mg, 0.03mmol, 0.03eq), ibrutinib (0.44g, 1mmol, 1.0eq), Li 2 CO 3 (88.7mg, 1.2mmol, 1.2eq), NMP (5mL), triisopropylsilanethiol (57.1mg, 0.3mmol, 0.3eq) and D 2 O (1 mL, 50 mmol, 50 equiv). The reaction mixture was irradiated for 36 hours at 30°C with a 25W 395nm blue LED light setting. The crude product was purified by flash chromatography to give a white solid (0.268 g, 60.22% yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com