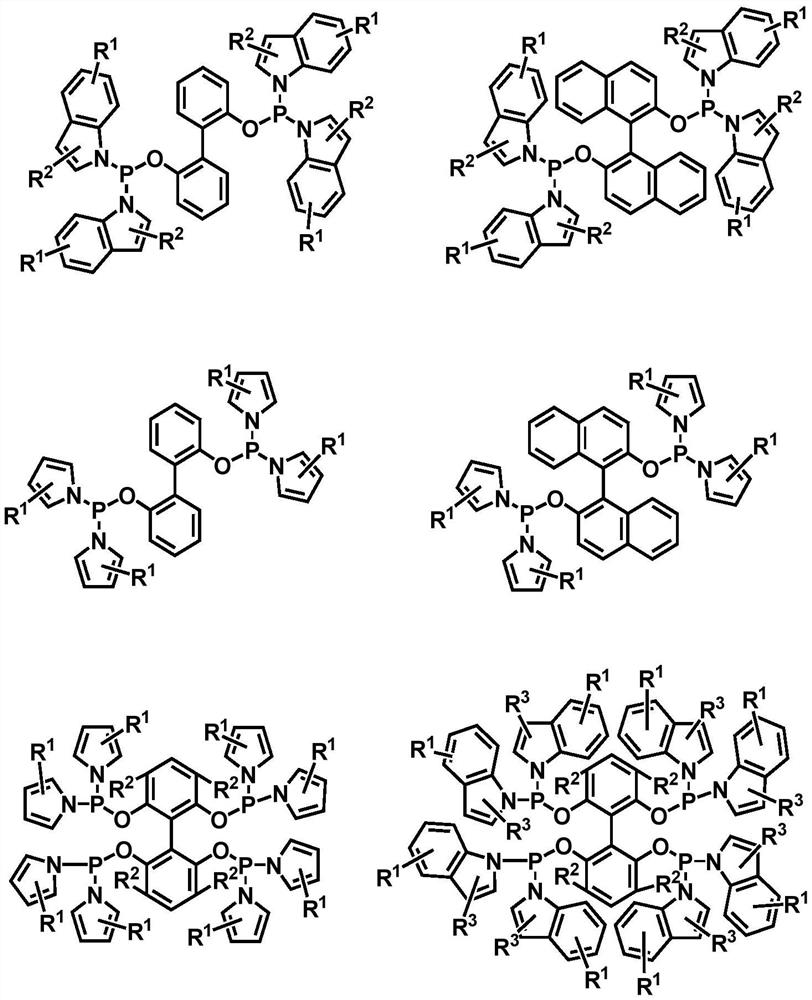
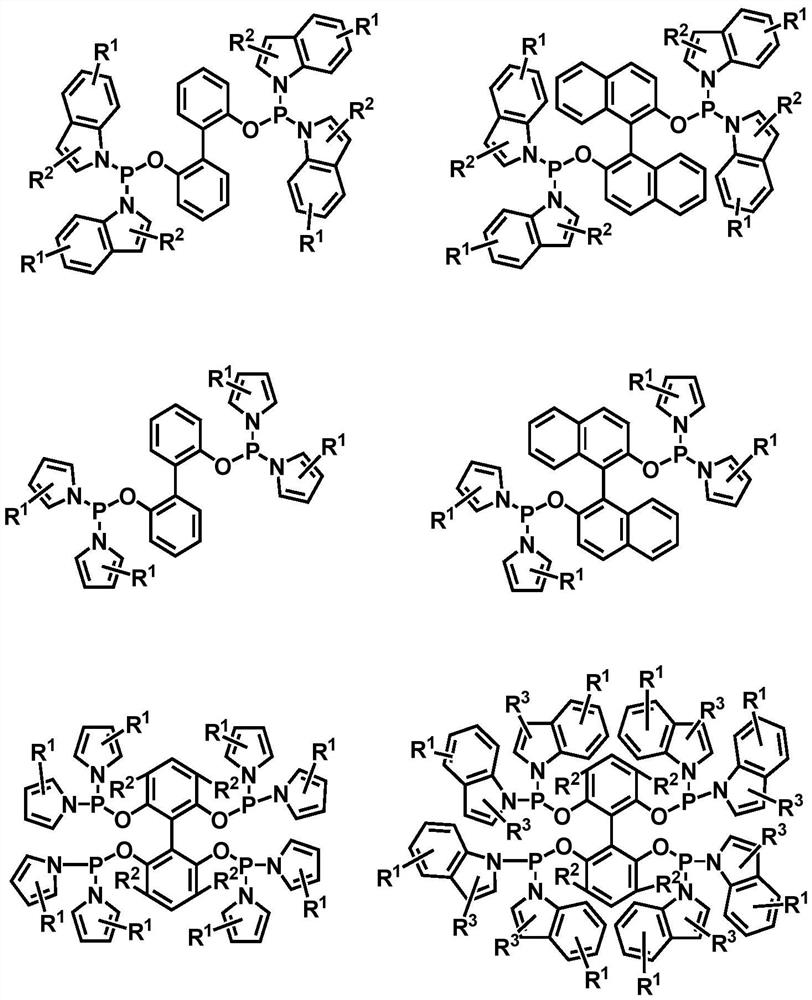
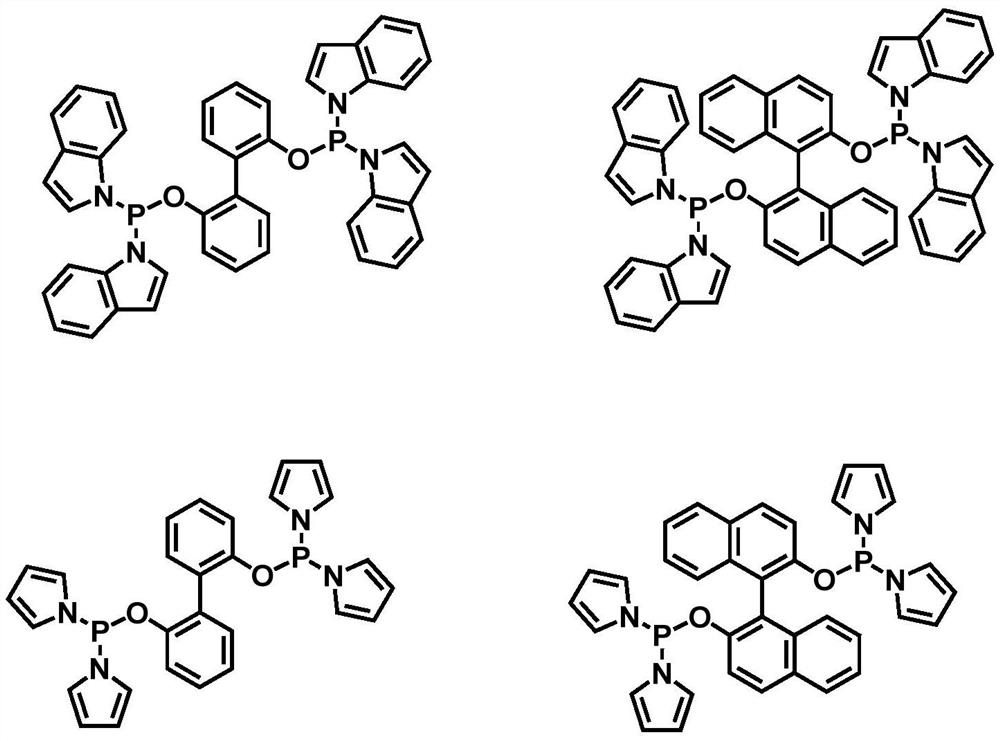
Method for preparing aldehyde by catalyzing internal olefin based on phosphoramidite phosphine ligand
A technology of phosphonamidite and internal olefin, which is applied in the field of preparing aldehydes based on phosphonamidophosphine ligands to catalyze internal olefins, can solve the problems of poor selectivity of aldehydes, ligand inactivation, harsh reaction conditions, etc., to reduce production energy consumption, Increased safety, pressure stabilization effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The invention provides a kind of method based on phosphinoamidophosphine ligand and rhodium catalyst catalyzed internal olefin to prepare linear valeraldehyde, comprising the following steps:
[0034] The phosphine ligand binaphthol bisindolyl phosphine and the metal rhodium precursor Rh(acac)(CO) 2 Put it into the autoclave with a molar ratio of 2:1, and then add toluene solvent, use the synthesis gas mixed with hydrogen and carbon monoxide at a partial pressure ratio of 1:1 to perform gas replacement operation, and then mix 5g of 2-butene with Rh (acac)(CO) 2 Samples were taken at a molar ratio of 1064 and added to the reactor. Then, under the condition of 1.0MPa (constant pressure) and 80°C, the reaction was stirred for 2h. After the reaction was completed, the product mixed solution was analyzed by gas chromatography, and the ratio of normal structure and isomerism valeraldehyde is as follows:
[0035]
[0036] a the molar ratio of normal aldehyde and isomeric...
Embodiment 2
[0039] The invention provides a kind of method based on phosphinoamidophosphine ligand catalyzed internal olefin linear valeraldehyde, comprising the following steps:
[0040] The phosphine ligand binaphthol bisindolyl phosphine and the metal rhodium precursor Rh(acac)(CO) 2 Add it to the autoclave with a molar ratio of 5:1, then add xylene solvent, use hydrogen and carbon monoxide in a partial pressure ratio of 2:1 to carry out gas replacement operation, and then mix 5g of 2-butene with Rh (acac)(CO) 2Samples were taken at a molar ratio of 975 and added to the kettle. Then, under the condition of 1.0MPa (constant pressure) and 70°C, the reaction was stirred for 6h. After the reaction was completed, the product mixed solution was analyzed by gas chromatography, and the ratio of normal structure and isomerism valeraldehyde is as follows:
[0041]
[0042] a is the molar ratio of normal aldehyde and isomeric aldehyde;
[0043] b is the total yield of valeraldehyde.
Embodiment 3
[0045] The invention provides a method for preparing linear valeraldehyde based on a phosphinoamidophosphine ligand catalyzed by an internal olefin, comprising the following steps:
[0046] Add the phosphine ligand binaphthol bisindolyl phosphine and metal rhodium precursor Rh(COD)(acac) in a molar ratio of 10:1 into the autoclave, then add trimethylbenzene solvent, press hydrogen and carbon monoxide to The mixed synthesis gas with a partial pressure ratio of 0.8:1 was used for replacement gas operation, and then 5 g of 2-butene and Rh(COD)(acac) were sampled at a molar ratio of 2000 and added to the reactor. Then, under the condition of 1.5MPa (constant pressure) and 80°C, the reaction was stirred for 6h. After the reaction was completed, the product mixed solution was analyzed by gas chromatography, and the ratio of normal structure and isomerism valeraldehyde is as follows:
[0047]
[0048]
[0049] a is the molar ratio of normal aldehyde and isomeric aldehyde;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com