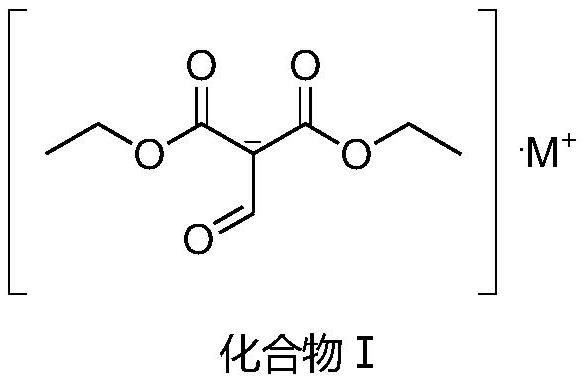
Preparation method of diethyl ethoxy methylene malonate
A technology of diethyl ethoxymethylene malonate and diethyl malonate is applied in the field of preparation of diethyl ethoxymethylene malonate, and can solve the problems of high raw material cost and reaction process. complex, harsh reaction conditions, etc., to achieve the effect of easy operation and realization, good atom economy, and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 68g of sodium ethoxide, 160g of diethyl malonate, 300g of ethanol, and 0.25g of piperidine into the autoclave, start stirring, feed carbon monoxide into the autoclave, ventilate three times, heat, and control the temperature at 100°C. Reach 2MPa, react for 2 hours. After the reaction, the temperature was lowered to room temperature, and the pressure was slowly released to normal pressure to obtain suspension I. Drop the suspension I into 300g of 30% ethanol hydrogen chloride for acidification, and stir at room temperature for 4 hours. Neutralize, filter, and distill under reduced pressure to obtain compound II with a yield of 87.1% and a purity of 98%.
Embodiment 2
[0038] Add 68g of sodium ethoxide, 160g of diethyl malonate, 300g of ethanol, and 0.5g of tetrabutylammonium bromide into the autoclave, start stirring, feed carbon monoxide into the autoclave, ventilate three times, and heat to control the temperature At 100°C, the pressure reached 2MPa, and the reaction was carried out for 2 hours. After the reaction, the temperature was lowered to room temperature, and the pressure was slowly released to normal pressure to obtain suspension I. The suspension I was dropped into 300 g of 30% ethanol hydrogen chloride for acidification, and stirred at room temperature for 4 hours. Neutralization, filtration, and distillation under reduced pressure gave Compound II with a yield of 84.7% and a purity of 97%.
Embodiment 3
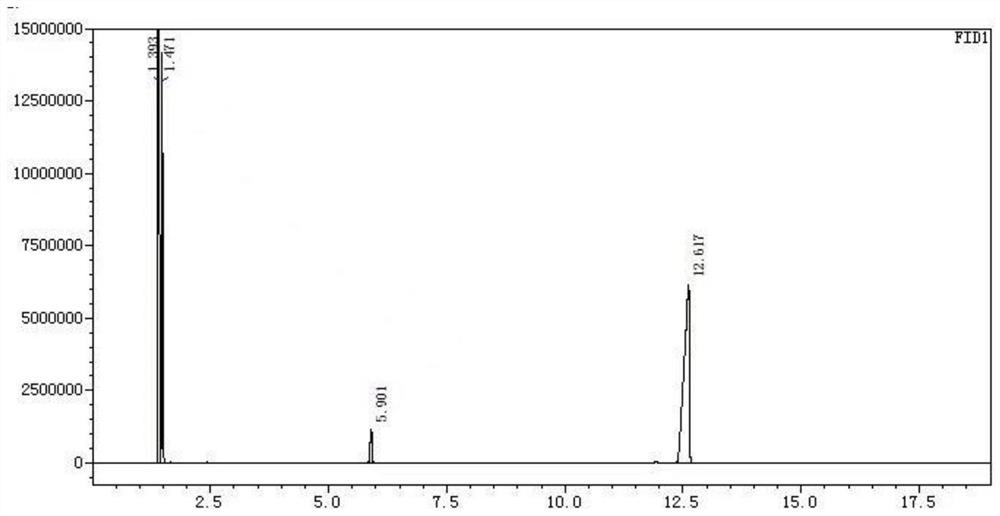
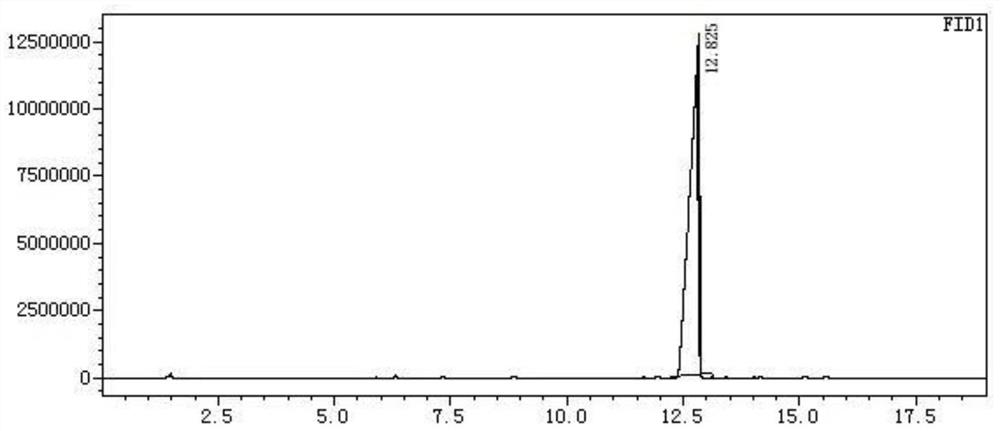
[0040] Add 68g of sodium ethoxide, 160g of diethyl malonate, 300g of ethanol, and 0.25g of dibenzo-24-crown-8 into the autoclave, start stirring, pass carbon monoxide into the autoclave, ventilate three times, and heat , the temperature was controlled at 100° C., the pressure reached 2 MPa, and the reaction was carried out for 2 hours. After the reaction, the temperature was lowered to room temperature, and the pressure was slowly released to normal pressure to obtain suspension I. The suspension I was dropped into 300 g of 30% ethanol hydrogen chloride for acidification, and stirred at room temperature for 4 hours. Neutralization, filtration, and vacuum distillation gave Compound II with a yield of 91.8% and a purity of 99%. The GC chart of the reaction solution is attached figure 1 As shown, the corresponding data are shown in Table 1, where the retention time is 5.901 for diethyl malonate, and the retention time is 12.617 for the product. The gc diagram of the purified pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com