Acridine compound-labeled steroid hormone derivative as well as preparation method and application thereof
A technology of steroid hormones and derivatives, applied in chemical instruments and methods, chemiluminescence/bioluminescence, biological testing, etc., can solve problems such as large steric hindrance, large batch-to-batch variation, and poor product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
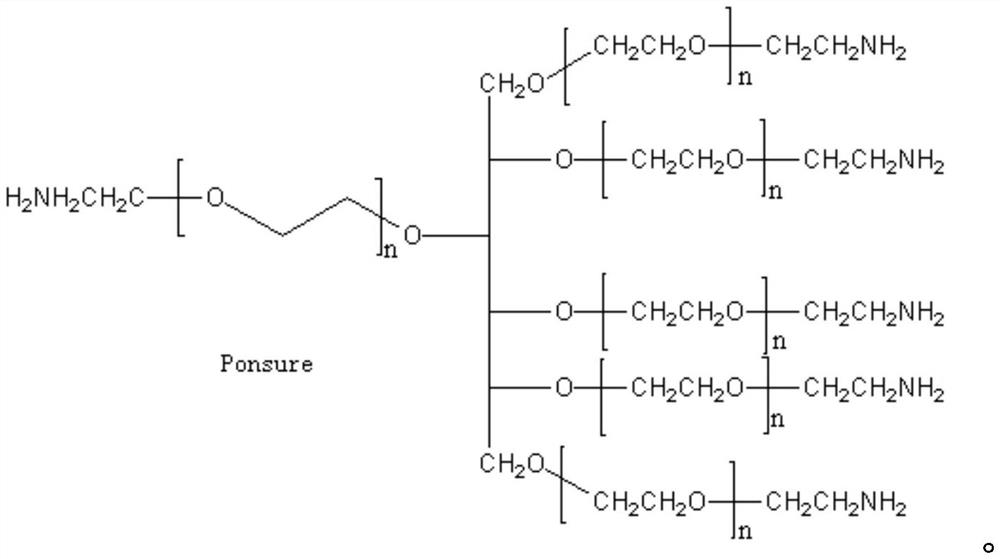
[0051] In a preferred embodiment, taking progesterone as an example, the carboxyl derivative group is carboxymethyl oxime, the modified polyethylene glycol is amino polyethylene glycol amino, and the acridine compound is acridinium ester DMAE-NHS. Specifically Preparation methods include:
[0052] (a) Dissolve progesterone-3-(O-carboxymethyl)oxime in dimethylformamide (DMF) at a concentration of 10 mg / ml, take 100 μl, add EDC and NHS, EDC and NHS were dissolved in 0.1M PBS pH7.2 at a concentration of 10mg / ml. React at room temperature for 15-30 minutes, add aminopolyethylene glycol amino (NH 2 -PEG-NH 2 ), aminopolyethylene glycol amino was dissolved in 0.1M PBS pH7.2 buffer, and reacted at room temperature for 1-2 hours.
[0053] (b) Dissolve acridinium ester DMAE-NHS in DMF at a concentration of 10 mg / ml, and add 1-10 times the molar amount of progesterone-3-(O-carboxymethyl)oxime to the solution after (a) reaction DMAE-NHS, reacted at room temperature for 1-2 hours, add...
Embodiment 1
[0058] A method for preparing acridinium ester-labeled progesterone derivatives, specifically:
[0059] (a) Dissolve progesterone-3-(O-carboxymethyl)oxime in dimethylformamide (DMF) at a concentration of 10 mg / ml, take 100 μl, add EDC and NHS, EDC and NHS were dissolved in 0.1M PBS pH7.2 at a concentration of 10mg / ml. React at room temperature for half an hour, add aminopolyethylene glycol amino (NH 2 -PEG-NH 2 , with an average molecular weight of 5000), aminopolyethylene glycol amino was dissolved in 0.1M PBS pH7.2 buffer, and reacted at room temperature for two hours.
[0060] (b) acridinium ester DMAE-NHS is dissolved in DMF, and concentration is 10mg / ml, adds 5 times of progesterone-3-(O-carboxymethyl) oxime molar amount to the solution that step (a) has reacted DMAE-NHS, react at room temperature for two hours, add 10 μl 1M glycine, react at room temperature for half an hour, and finally use 0.1M PBSPH7.2 buffer solution to make the reaction solution equal to the mass...
Embodiment 2
[0062] A method for preparing acridinium ester-labeled progesterone derivatives, specifically:
[0063] (a) Amino polyethylene glycol amino (NH 2 -PEG-NH 2 , number average molecular weight is 5000), dissolved in 0.1M PBS PH7.2 buffer solution, the concentration is 1mg / ml, take 1ml, add DMAE-NHS of 1.5 times the molar amount of aminopolyethylene glycol amino, DMAE-NHS is dissolved in In DMF, the concentration is 10mg / ml, and react at room temperature for half an hour.
[0064] (b) Dissolve progesterone-3-(O-carboxymethyl)oxime in dimethylformamide (DMF) at a concentration of 10 mg / ml, add EDC and NHS in a molar ratio of 1:1:1.2, EDC And NHS dissolved in 0.1M PBSPH7.2 buffer, the concentration is 10mg / ml. Add a mixed solution of progesterone-3-(O-carboxymethyl)oxime in the same molar amount as the aminopolyethylene glycol amino group to the solution that has been reacted in step (a), react at room temperature for two hours, add 10 μl of 1M glycine, and React for half an hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com