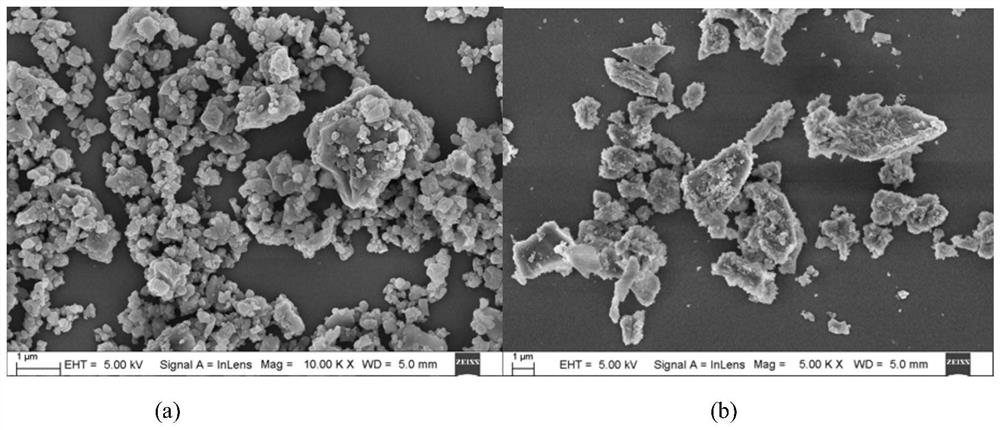
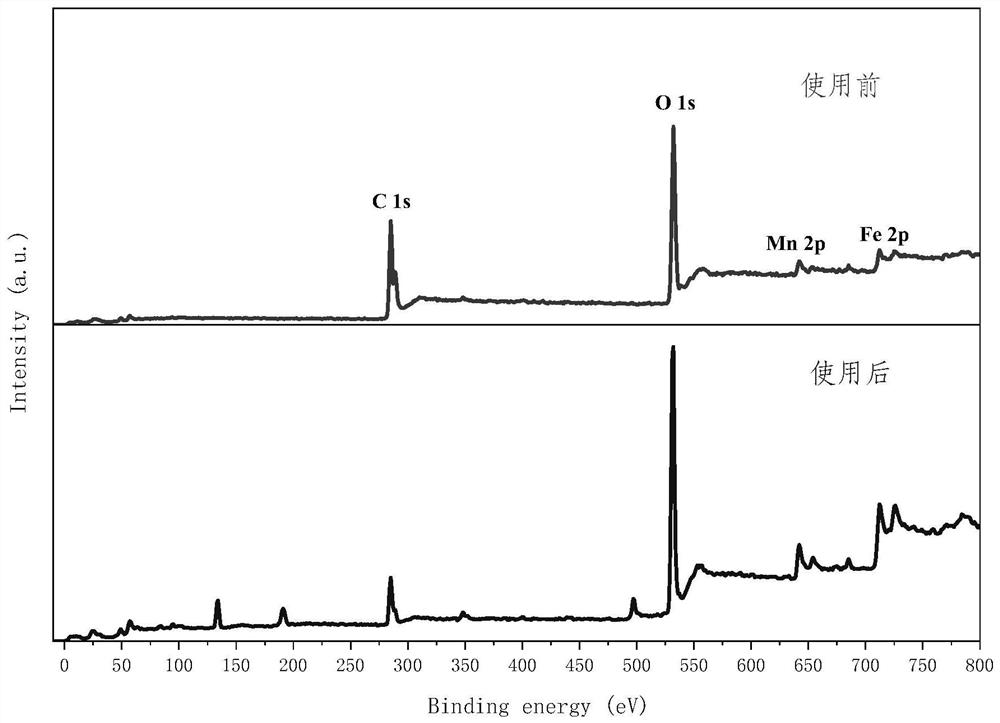
Bimetallic organic framework material catalyst, preparation method and application
An organic framework and catalyst technology, applied in the field of catalytic reduction of precursor ozonation to generate nitrogen-containing by-products, preparation, bimetal organic framework material catalyst field, can solve the problems of MOFs catalyst characteristics, unclear synthesis method, etc., to achieve the reduction effect Excellent, high reduction rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A method for preparing a bimetallic organic framework material catalyst, comprising the steps of:
[0032] (1) Synthesis: 0.3350g zero-valent iron, 2.1470g Mn(NO 3 ) 2 Solution (50wt%), 2.5220g H 3 BTC, 600μL hydrofluoric acid (100%) and 75ml H 2 O was placed in a 100 mL reaction kettle lined with tetrapolyethylene fluoride, stirred and mixed evenly with a tetrafluoroethylene stirring rod, heated at 120 °C for 2 h, and then heated to 150 °C for 4 h.
[0033] (2) Washing: Take out the product and filter it with suction, soak the obtained product in absolute ethanol for 12 hours, and then wash it 5 times with fresh absolute ethanol;
[0034] (3) Drying: the orange product was separated by suction filtration, and the product was dried at a constant temperature of 70° C. for 12 hours.
[0035] (4) Vacuum activation: the dried powder product was then vacuum activated at a constant temperature of 200° C. for 12 hours to finally obtain the desired catalyst.
[0036] Subse...
Embodiment 2
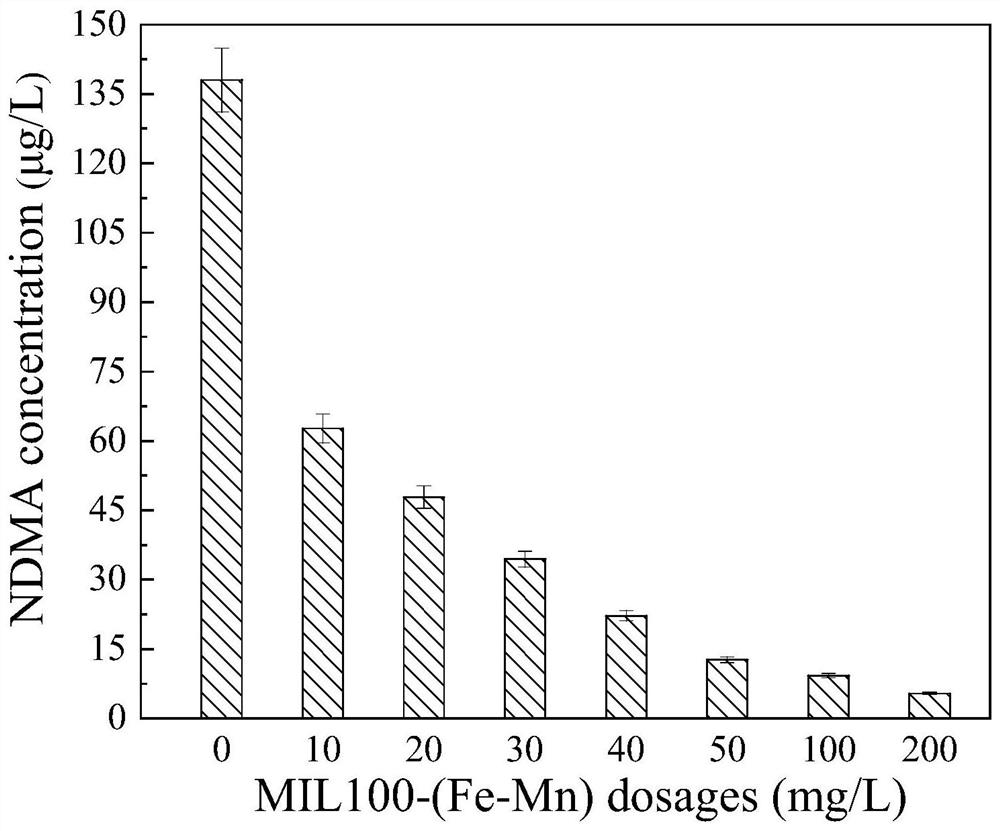
[0040] The step (1)~(4) catalyst preparation method of embodiment 2 is the same as embodiment 1, and the difference with embodiment 1 is: the NDMA generation situation of catalyzed ozonation diethylhydrazide by different ozone concentrations, comprises the following steps:
[0041] Add ozone concentrations of 0, 0.5, 1, 2, and 4 mg / L to the samples containing 20 μM diethylhydrazide, and carry out catalytic oxidation for 1 hour under the conditions of pH 7 and catalyst dosage of 200 mg / L. Use 80 g / L sodium thiosulfate solution for quenching to terminate the reaction, respectively detect the generation of NDMA after the reaction.
[0042] The result is as Figure 4 , it can be seen that the formation of NDMA increases with the increase of ozone concentration in the process of catalytic ozonation and ozonation alone, but it can be clearly seen that at different ozone concentrations, the formation of NDMA is significantly reduced by catalytic ozonation.
Embodiment 3
[0044]The step (1)~(4) catalyst preparation method of embodiment 3 is the same as embodiment 1, and the difference with embodiment 1 is: by the NDMA generation situation of catalyzed ozonation diethylhydrazide under different pH, comprise the following steps:
[0045] A series of samples containing 20μM diethylhydrazide were adjusted to 5, 6, 7, 8, 9 respectively, the dosage of ozone was 2mg / L, and the dosage of catalyst was 200mg / L, and the catalytic oxidation was carried out for 1h. L sodium thiosulfate solution was quenched to terminate the reaction, and the generation of NDMA after the reaction was detected respectively.
[0046] The result is as Figure 5 , it can be seen that when butylhydrazide is oxidized by ozone alone, the formation of NDMA first increases and then decreases with the increase of pH, and reaches the maximum value at pH 8; while when bimetallic organic framework catalyzes ozone, the formation of NDMA increases with the increase of pH The increase firs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com