Method for preparing 2-amino-4-(methylthio)butyramide
A technology of methylthiobutyramide and aminobutyronitrile is applied in sulfide preparation, chemical recovery, organic chemistry and other directions, and can solve the problems of easy polymerization of nitrile, and achieve the effects of difficult polymerization, high yield and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
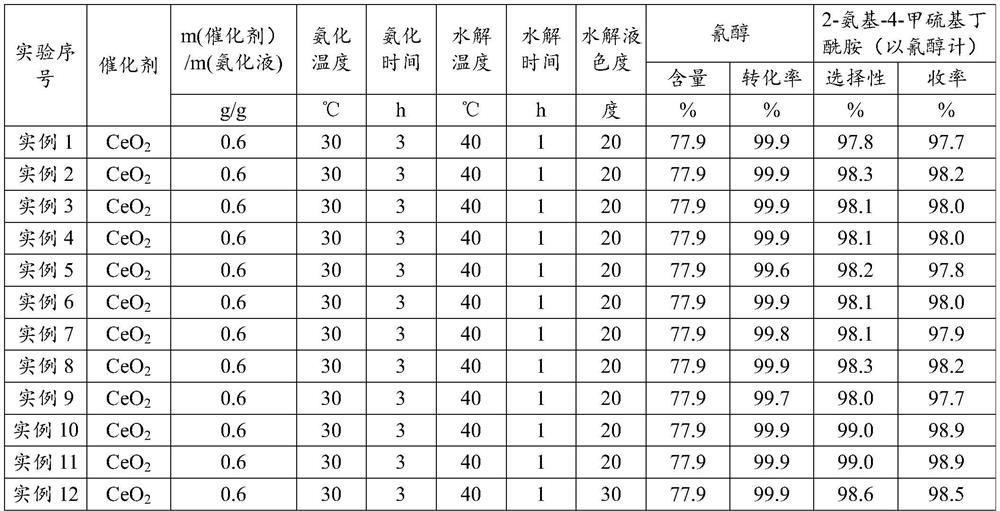
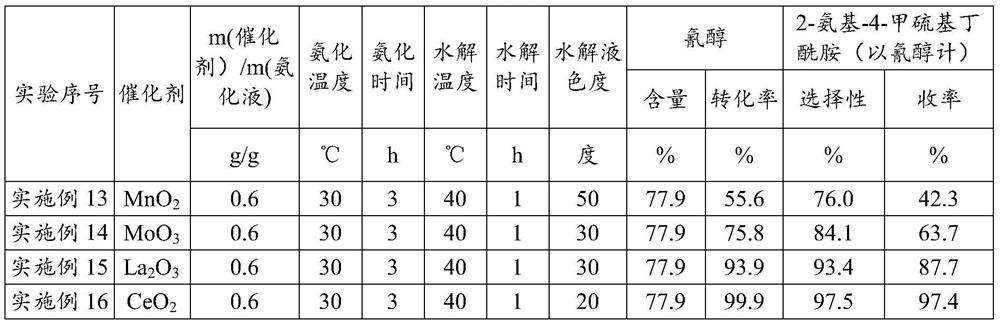
[0030] Embodiment 1 (ceria is made catalyst, the 1st batch)
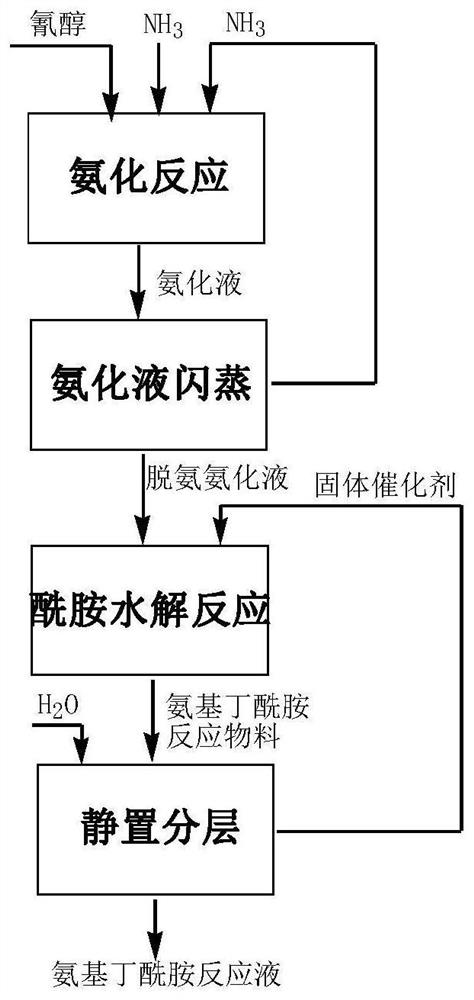
[0031]Put into cyanohydrin (60g77.9%, 0.356mol) in the stainless steel autoclave that is equipped with magnetic stirrer and jacket, under the state that is heated to 30 ℃, pass into ammonia gas, control pressure 0.3-0.4MPa, at 30 Stir at ℃ for 3 hours, the consumption of ammonia is 3.5 times of the moles of cyanohydrin, and the obtained ammoniated solution containing aminobutyronitrile is flashed to remove excess ammonia, and the ammonia content is 6.3%. After deamination, add 56.7g of ammoniated solution containing aminobutyronitrile and 35g of solid catalyst cerium oxide into the reaction device, maintain 40°C for hydrolysis reaction, and react to generate 2-amino-4-methylthiobutyramide, hydrolysis time 1h . After completion of the reaction, add 390g of water, keep the heat preservation and let it stand for 0.5h to stratify. The catalyst at the bottom of the reaction device is applied mechanically to the next bat...
Embodiment 2
[0032] Embodiment 2 (ceria is made catalyst, and catalyst is applied mechanically the 1st batch)
[0033] Put into cyanohydrin (60g77.9%, 0.356mol) in the stainless steel autoclave that is equipped with magnetic stirrer and jacket, under the state that is heated to 30 ℃, pass into ammonia gas, control pressure 0.3-0.4MPa, at 30 Stir at ℃ for 3 hours, the consumption of ammonia is 3.5 times of the moles of cyanohydrin, and the obtained ammoniated solution containing aminobutyronitrile is flashed to remove excess ammonia, and the ammonia content is 5.8%. After deamination, 56.6 g of the ammoniated solution containing aminobutyronitrile was added to the reaction device containing ceria catalyst, and the hydrolysis reaction was carried out at 40 °C to generate 2-amino-4-methylthiobutyramide, and the hydrolysis time was 1 hour. After the reaction is complete, add 390g of water, keep the heat and let it stand and separate for 0.5h. The catalyst at the bottom of the reaction device i...
Embodiment 3
[0034] Embodiment 3 (ceria is made catalyst, and catalyst is applied mechanically the 2nd batch)
[0035] Put into cyanohydrin (60g77.9%, 0.356mol) in the stainless steel autoclave that is equipped with magnetic stirrer and jacket, under the state that is heated to 30 ℃, pass into ammonia gas, control pressure 0.3-0.4MPa, at 30 Stir at ℃ for 3 hours, the consumption of ammonia is 3.5 times of the moles of cyanohydrin, and the obtained ammoniated solution containing aminobutyronitrile is flashed to remove excess ammonia, and the ammonia content is 5.9%. After deamination, 57.2 g of the ammoniated solution containing aminobutyronitrile was added to the reaction device containing ceria catalyst, and the hydrolysis reaction was carried out at 40°C to generate 2-amino-4-methylthiobutyramide, and the hydrolysis time was 1 hour. After the reaction is complete, add 390g of water, keep the temperature and let it stand for 0.5h to separate layers. The catalyst at the bottom of the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com