Synthesis method of chiral amine
A synthesis method and technology of chiral amines, applied in the field of chiral amine synthesis, can solve the problems of harsh reaction conditions and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Screening of wild-type amino enzymes
[0054] This embodiment first chooses to Chromobaterium Violaceum The transaminase (sequence such as SEQID NO: 1, which is recorded as a CVTA wild type aminotransferase), the catalytic activity of the substrate (substrate 1) which catalyzes the primary resistance of the synthesis of the target is tested. Among them, the detailed process of the CVTA wild-type amino enzyme catalytic substrate 1 is as follows: 1 ml system includes 1 mg substrate 1, 0.1 mg PLP, 1 mg isopropylamine hydrochloride, 50 mg enzyme powder, pH 8.0 100 mm phosphate buffer Liquid, 30 o C reaction 40 h.
[0055] The results show that the efficiency of the CVTA wild-type amino enzyme catalytic synthetic substrate 1 is 0.56%.
[0056] Next, it is selected that the same but different kinds of the above CVTA wild-type aminaminase, and the amino acid sequence is selected from the SEQ IDNO: 1, and the homology is from 82% of the wild-type aminaminase (specifically...
Embodiment 2
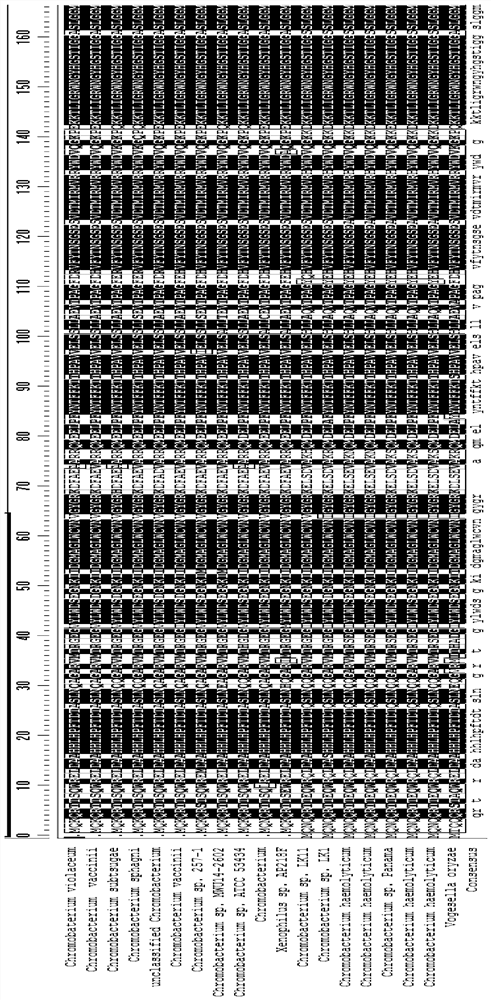
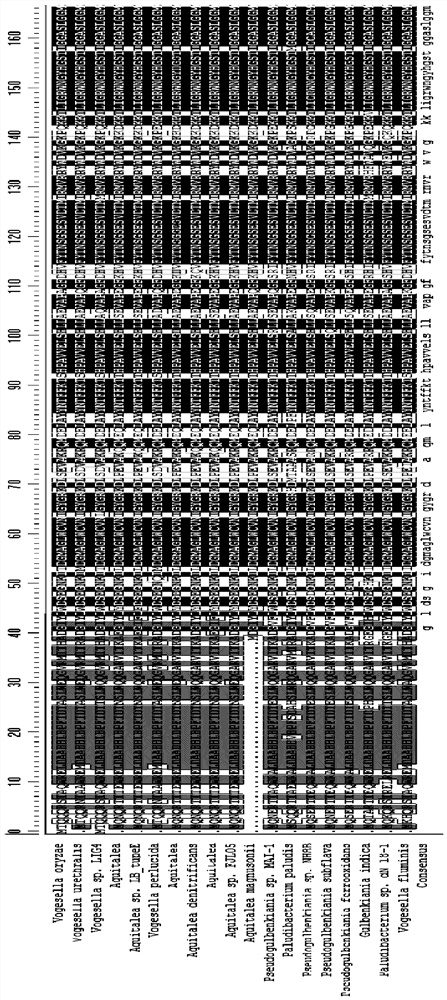
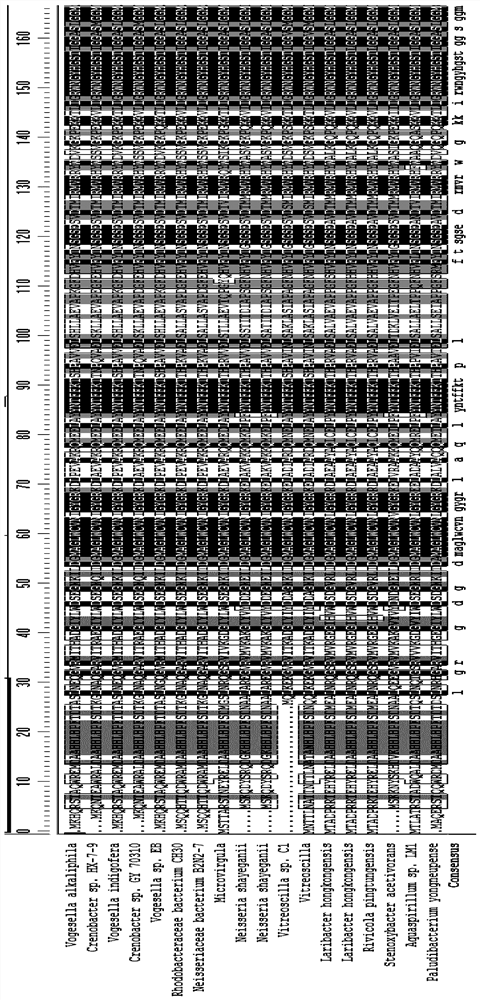
[0062] In addition to the transaminase from the Chromobacterium source, this application also tested other stems of 69% to 87% homology with ChromOBATERIUMVIOLACEUM. The specific information is shown in Table 2.
[0063] The wild-type aminotransferase of different sources in this embodiment, the amino acid sequence between the 54th and 63th and the 96th and the 96th, also shows highly conservative. These wild-type transaminases have been tested to have a catalytic activity of 0.01% to 1% for the target large-site resistant substrate (substrate 1) (see Table 2).
[0064] Table 2: With CHROMOBATERIUM VIOLACEUM, it has lower homology, but derived from other transaminase.
[0065]
[0066] .
[0067] In the above table, + represents the conversion rate of 0.01% to 0.1%, ++ represents between 0.1 to 0.5%, ++ + represents the conversion rate of 0.5 ~ 1%.
[0068] Further, the sequence alignment is performed on the different aminaminase strains shown in Tables 1 and 2, and the compar...
Embodiment 3
[0070] In order to further confirm that the impact of the aminotransferase of the above conservative amino acid region, the inventor is preliminary Chromobaterium Violaceum The conservative amino acid region 1 in the source of the source of transaminase (ie, SEQ ID NO: 1) (specific sequence is DGMAGL) WC The amino acid W and C in VnVGYGR were mutated, and the catalytic efficiency of these conserved amino acids had improved after mutation of these conservative amino acids, and the conversion rate after WA mutation was increased between 5 and 10%, and CA mutation The conversion rate is increased to between 1 to 5% (the specific reaction process is described in Example 1).
[0071] In order to confirm the source of transaminase from other species, the conversion of the substrate has increased after the conserved site, and the inventors also have the same mutation of other sources of transaminase, and the test results are shown below.
[0072] table 3:
[0073] .
[0074] In the abo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com