Nickel-based catalyst for methane carbon dioxide dry reforming as well as preparation method and application of nickel-based catalyst
A nickel-based catalyst, carbon dioxide technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the weak interaction between active metal and support, catalytic performance problems such as loss, complex modification methods, etc., to achieve excellent methane dry reforming activity and stability, simple preparation steps, and overcome the effects of carbon deposition and sintering problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
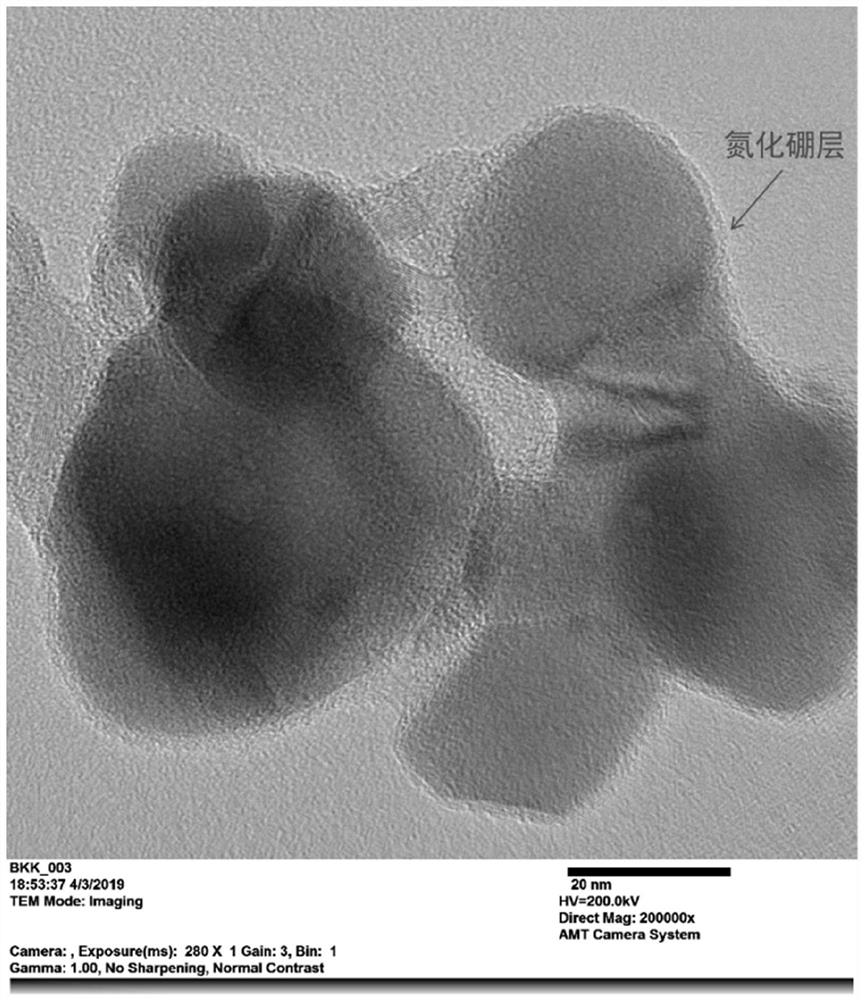
[0030] A nickel-based catalyst for methane carbon dioxide dry reforming reaction, with metal oxide ZrO 2 As a carrier, metal nickel is supported, followed by boron nitride coating, so that a boron nitride protective layer is coated on the surface of the oxide-supported nickel-based catalyst to form a nickel-based catalyst composite material for methane carbon dioxide dry reforming reaction.
[0031] A method for preparing a nickel-based catalyst used in this embodiment for methane carbon dioxide dry reforming reaction, the process steps are as follows:
[0032] a. The first stage of calcination:
[0033] 0.55g Ni(NO 3 ) 2 ·6H 2 O and 1.0 g nanometer ZrO 2 Pour the powder into a round-bottomed flask, then add 80mL of deionized water, mix it evenly with ultrasound, and after stirring thoroughly, evaporate to dryness with a rotary evaporator at 45°C to remove the solvent of the suspension, collect the powder sample and place it in Dry overnight in a blast oven at 60°C; then ...
Embodiment 2
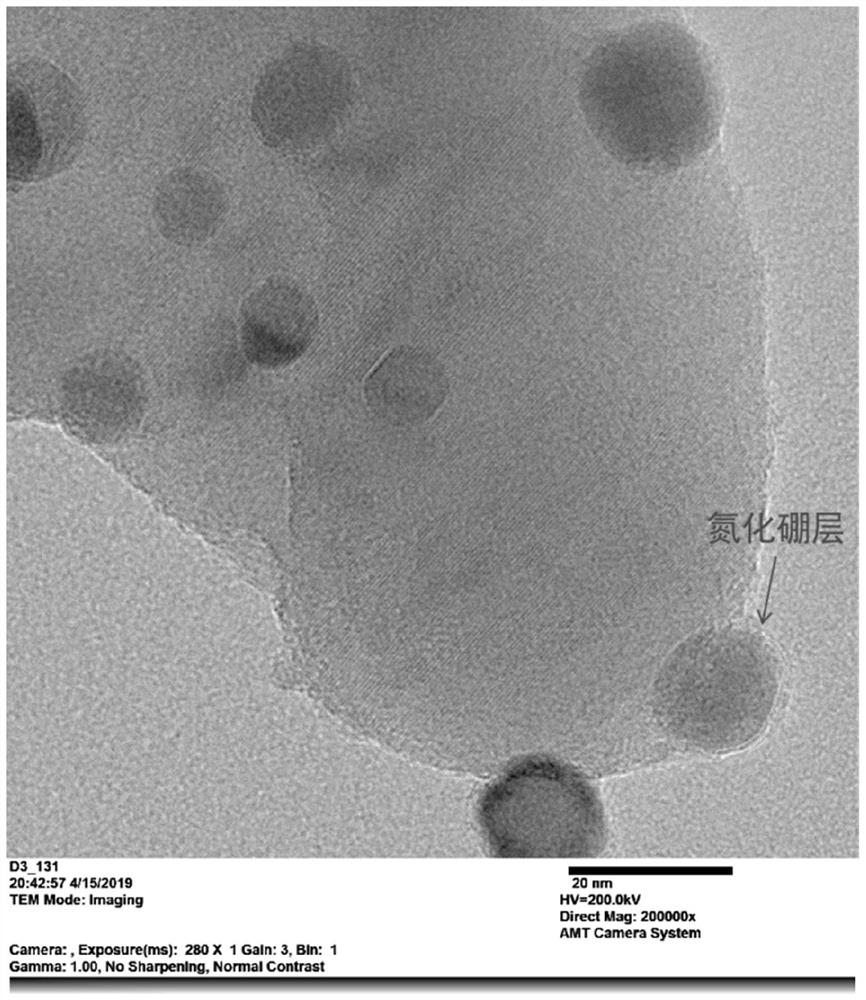
[0042] A nickel-based catalyst for the dry reforming reaction of methane and carbon dioxide. The metal oxide lanthanum oxide is used as a carrier, and the nickel-based catalyst is coated with nitrogen on the surface of the oxide-supported nickel-based catalyst by loading metal nickel and then coating with boron nitride. A boron oxide protective layer is used to form a nickel-based catalyst composite for methane carbon dioxide dry reforming reaction.
[0043] A method for preparing a nickel-based catalyst used in this embodiment for methane carbon dioxide dry reforming reaction, the process steps are as follows:
[0044] a. The first stage of calcination:
[0045] 0.55gNi(NO 3 ) 2 ·6H 2 Pour O and 1.0g of nanometer lanthanum oxide powder into a round bottom flask, then add 80mL of deionized water, mix evenly by ultrasonic, after fully stirring, evaporate to dryness with a rotary evaporator at 45°C, and remove the solvent of the suspension , collect the powder samples and pu...
Embodiment 3
[0054] A nickel-based catalyst for methane carbon dioxide dry reforming reaction, with metal oxide ZrO 2 As a carrier, metal nickel is supported, followed by boron nitride coating, so that a boron nitride protective layer is coated on the surface of the oxide-supported nickel-based catalyst to form a nickel-based catalyst composite material for methane carbon dioxide dry reforming reaction.
[0055] A method for preparing a nickel-based catalyst used in this embodiment for methane carbon dioxide dry reforming reaction, the process steps are as follows:
[0056] a. The first stage of calcination:
[0057] 0.47gNi(CH 3 COO) 2 4H 2 O and 1.0 g nanometer ZrO 2 Pour the powder into a round-bottomed flask, then add 80mL of deionized water, mix it evenly with ultrasound, and after stirring thoroughly, evaporate to dryness with a rotary evaporator at 45°C to remove the solvent of the suspension, collect the powder sample and place it in Dry overnight in a blast oven at 60°C; then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com