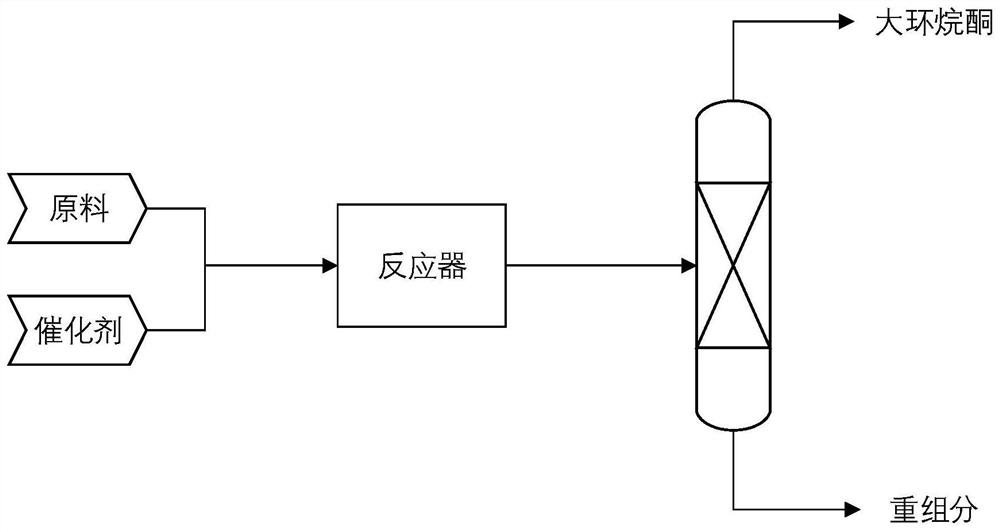
Method for preparing macrocycloalkanone from epoxide
A technology for epoxide and macrocycloalkanone, applied in the field of chemical synthesis technology, can solve the problems of undisclosed catalyst recovery, high price of lithium bromide, lower production cost, etc., achieves low price, obvious energy consumption advantage, reduced difficulty and operation effect of difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
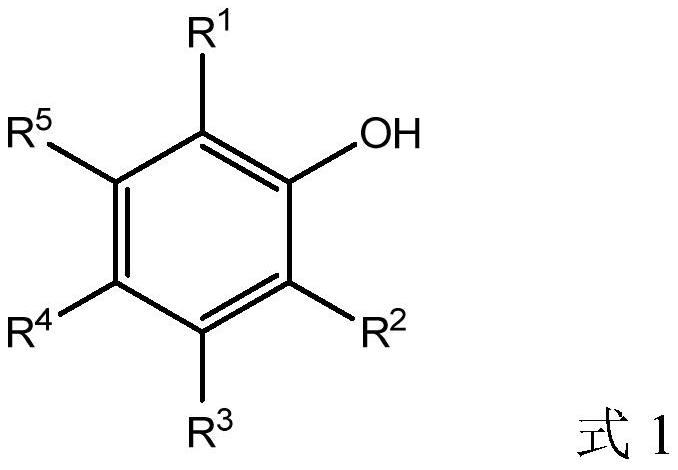
[0052] Isomerization of 1,2-epoxycyclododecane to cyclododecanone in the presence of a catalyst prepared from ligand L1 (2,6-diphenylphenol).
[0053] Add 10.5 mmol of ligand L1 (2,6-diphenylphenol) and 100 mL of anhydrous toluene into a heat-dried reaction flask. At room temperature, 6.6 mmol of trimethylaluminum-toluene solution (0.2 mol / L) was added to the transparent solution. The solution was stirred at 25°C. After a few minutes, a catalyst solution was obtained. The catalyst solution was heated to 40°C, and 120 g (659 mmol) of 1,2-epoxycyclododecane was added within 2 h, and the temperature was maintained to continue stirring for 2 h. After the reaction solution was detected by gas chromatography and quantified by calibration and normalization method, the conversion rate of 1,2-epoxycyclododecane was 100%, and the selectivity of cyclododecanone was 98.3%. After the reaction was completed, the reaction solution was rectified and purified to finally obtain cyclododecano...
Embodiment 2
[0055] Isomerization of 1,2-epoxycyclooctane to cyclooctanone in the presence of a catalyst prepared from ligand L2 (2,6-di-tert-butylphenol).
[0056] Add 20 mmol of ligand L2 (2,6-di-tert-butylphenol) and 100 mL of anhydrous toluene into a heat-dried reaction flask. At 0°C, 6.6 mmol of triethylaluminum-toluene solution (0.2 mol / L) was added to the transparent solution. The solution was stirred at 0 °C. After a few minutes, a catalyst solution was obtained. The catalyst solution was heated to 20° C., and 167 g (1.32 mol) of 1,2-epoxycyclooctane was added within 1 h, and the temperature was maintained to continue stirring for 3 h. After the reaction solution was detected by gas chromatography and quantified by calibration and normalization method, the conversion rate of 1,2-epoxycyclooctane was 99.8%, and the selectivity of cyclooctanone was 99.2%. After the reaction was finished, the reaction liquid was subjected to rectification and purification, and finally the yield of ...
Embodiment 3
[0058] Isomerization of 1,2-epoxycyclodecane to cyclodecanone in the presence of a catalyst prepared from ligand L3 (2,6-diα-naphthylphenol).
[0059] Add 20mmol of ligand L3 (2,6-diα-naphthylphenol) and 100mL of anhydrous toluene into the heat-dried reaction flask. At 50°C, 10 mmol of tripropylaluminum-toluene solution (0.1 mol / L) was added to the transparent solution. The solution was stirred at 50°C. After a few minutes, a catalyst solution was obtained. The catalyst solution and the raw material 1,2-epoxycyclodecane were respectively pumped into a 3-stage series reactor, the jacket of the reactor was circulating water, the temperature inside the kettle was controlled at 60°C, and the reaction pressure was normal pressure. By controlling the flow rate of the pump, the molar ratio of the catalyst to the raw material 1,2-epoxycyclodecane is 0.001:1, and at the same time, the residence time of the reaction solution in each reactor is 15 minutes. The reaction continued to ru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com