T cell proliferation method and application thereof
A cell and cell suspension technology, applied in the field of 3D bioprinting T cell proliferation, can solve the problems of high degree of cell exhaustion, high cost of CAR-T cell preparation, and difficulty in obtaining T cells, and achieve the effect of improving survival rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
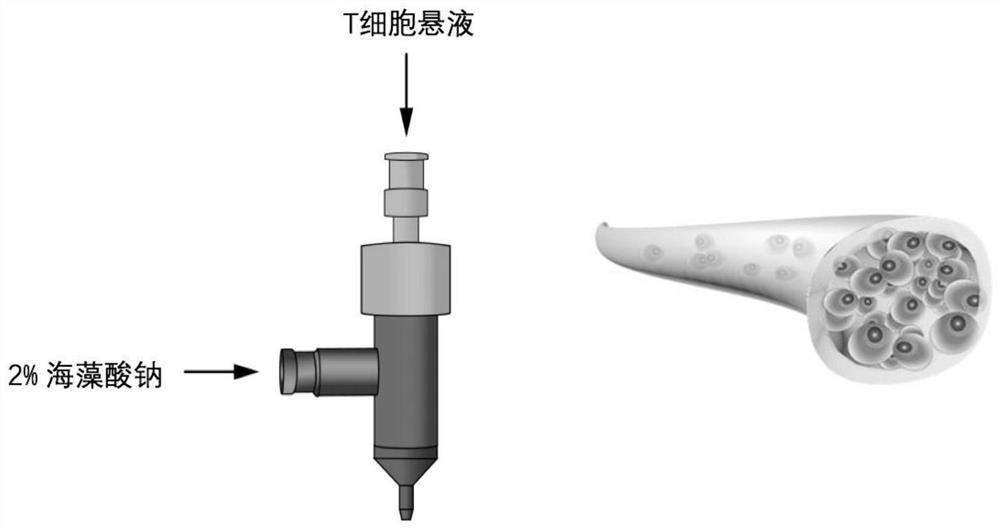
[0088] Example 1: Three-dimensional coaxial printing to obtain T cells
[0089] experimental method:
[0090] Take 20mL of healthy human blood, add it to a heparin anticoagulant tube, add 20mL of PBS to dilute, slowly add 10mL of Ficoll separation solution, and use a centrifuge to centrifuge at 2000r / min for 20 minutes; absorb the milky white layer after centrifugation, that is, PBMC, and then add an appropriate amount of PBS to wash , and finally add an appropriate amount of PBS for counting.
[0091] Next, wash the PBMC with an appropriate amount of MACS buffer, and continue to use MACS buffer to resuspend, add CD3 immunomagnetic beads (20μL / 10 7 PBMC) mix well, and incubate at 4°C for 15 minutes; wash the cells again with MACS buffer, resuspend and add to the washed MS separation column, the cells that flow out are CD3-T cells, wash the isolate 3 times with MACS buffer, and finally add 1mL of cells were eluted, and the eluate was CD3+T cells, which were counted and resusp...
Embodiment 2
[0098] Example 2: Obtaining T cells in suspension culture
[0099] The processing before printing is the same as that in Embodiment 1. T cells were divided into 1 x 10 6 / ml density inoculated in T25 culture flasks, and the solution was changed every three days.
Embodiment 3
[0100] Example 3: Comparison of proliferation ability of T cells obtained by three-dimensional coaxial printing and T cells obtained by suspension culture
[0101] experimental method:
[0102] In order to evaluate the proliferation ability of T cells in coaxial bioprinted T cell-loaded hydrogel fibers, we used a flow cytometer (Beckman, CytoFlex flow cytometer) to analyze the fluorescence intensity of CFSE in T cells (the fluorescence intensity was halved, the cells Division once), which represents the division algebra of T cells. On the 3rd, 7th, and 10th day after printing, we used citric acid-EDTA solution to dissolve the sodium alginate shell of the coaxial fiber, and collected quantitative cells to detect the fluorescence intensity of CFSE.
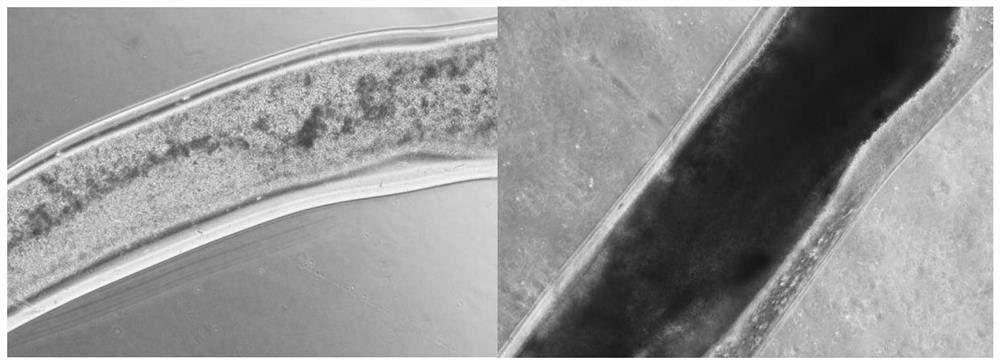
[0103] Experimental results:
[0104] Experimental results such as image 3 and Figure 4 shown.
[0105] Such as image 3 As shown, by observing the micrographs of the 3D coaxially printed T cells on the 3rd day (left) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com