A kind of supercapacitor based on gel polymer electrolyte and preparation method thereof
A gel polymer and supercapacitor technology, applied in the field of energy storage, can solve the problems of easy leakage, volatile liquid electrolyte, flammability, etc., achieve good low temperature tolerance, good electrochemical performance, and expand the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] The preparation method of the flame retardant gel polymer electrolyte according to the present invention includes the following steps:
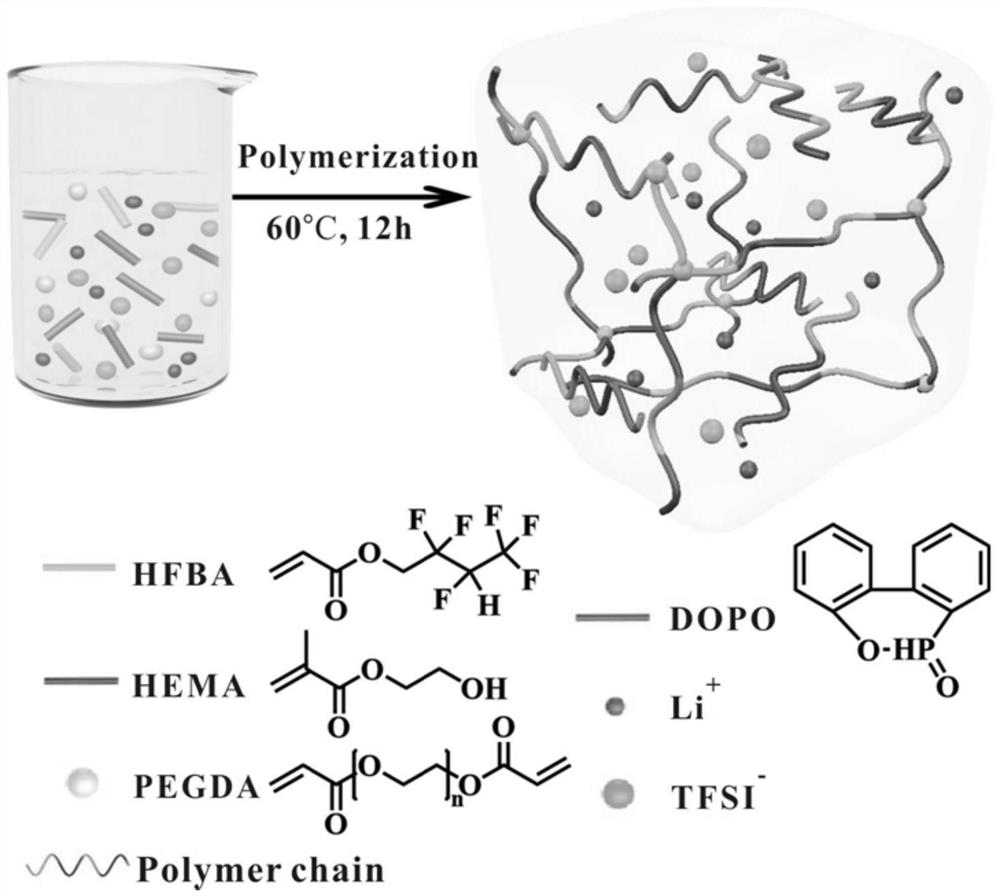
[0062] 1) Add acrylic hexafluorobutyate (HFBA) and methyl methacrylate (HEMA) to the solvent. The solvent is DMF, acetonitrile, DMSO and other organic solvents, and the solvent is preferably 50% -75%, and the solvent accounts for 50% -75% of the total weight of 50% to 75% by the total weight of the monomer and the solvent. The amount of solvent is used to have a relatively large effect on the conductivity. In step 1), the molar ratio of HFBA and HEMA is greater than 2: 1; its ratio can be high or even near infinity, such as HFBA and HEMA's molar ratio 1: 0; more preferred, HFBA and HEMA molar ratio ( 2 ~ 10): 1; More preferably, HFBA and HEMA molar ratios are 4 to 10: 1 (including 4: 1, 6: 1, 8: 1, 10: 1). More preferably, the molar ratio of HFBA and HEMA is 8: 1.
[0063] 2) A solution formed by the flame retardant, polyethylene glycol di...
Embodiment 1
[0065] Example 1 Preparation of gel polymer electrolyte
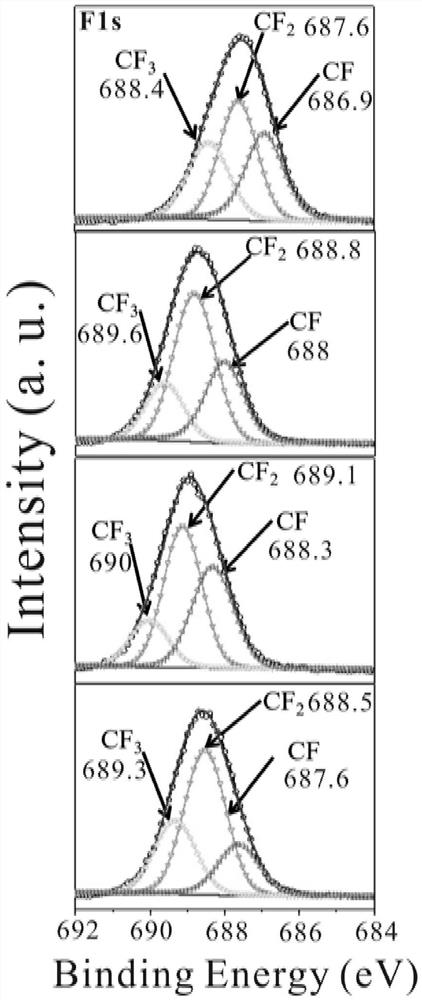
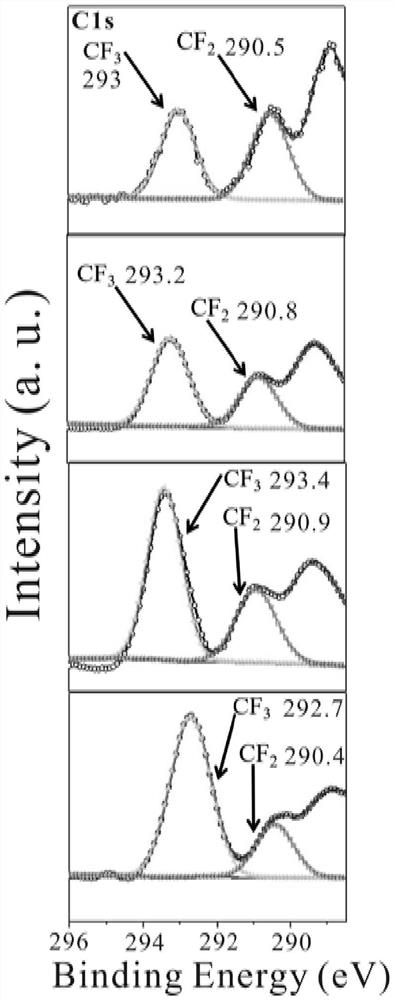
[0066] HFBA (acrylate hexafluorol) and HEMA (methyl methacrylate) were added to the DMF solvent in the ratio of molar ratio 6: 1 (the mass ratio of the monomer and the solvent was 4: 6). Then, the total mass of the monomer is 1% by weight of 9,10-dihydro-9-oxidation (DOPO), with respect to the total mass of the monomer with respect to the total mass of the monomer with respect to the total mass of the monomer. 1.5% by weight of polyethylene glycol diacrylate (PEGDA) and 2.5 mol L -1 Litfsi (4.306 g) was added to the above solution. Finally, the solution was transferred to the mold after the addition initiator (AIBN with a total of 2% of the total mass of the monomer). After 12 hours at 60 ° C, the resulting gel polymer electrolyte (abbreviated as poly (Hfbax-co-hemay) was obtained.
Embodiment 2-8
[0067] Example 2-8 Preparation of gel polymer electrolyte
[0068] HFBAs and HEMAs added to 20 ml of glass bottles will have different molar ratios (1: 0, 10: 1, 8: 1, 6: 1, 4: 1, 2: 1). The amount of solvent is used between 50% and 75%. Then, the DOPO (with respect to the total mass of the monomer is 1% by weight), and the total mass of the monomer is 1.5% by weight) and 0 to 3 mol L. -1 Litfsi is added to the above solution. Litfsi concentration is controlled in 0.5, 1, 1.5, 2, 2.5, 3mol L, respectively. -1 . Finally, the solution was transferred to the mold after the addition initiator (AIBN with a total of 2% of the total mass of the monomer). After 10-14 hours at 50-80 ° C, the resulting gel polymer electrolyte (abbreviated as poly (Hfbax-co-hemay), wherein X and Y represent HFBA and HEMA molar ratio. Reaction conditions Table 1.
[0069]Table 1 Preparation parameters of gel polymer electrolyte
[0070]
[0071] In a particular embodiment of the invention, the solvent, flam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com