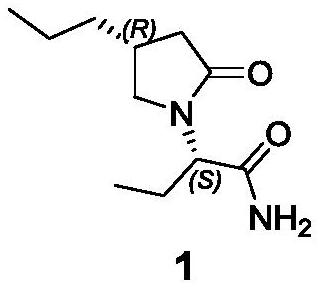
Preparation method of brivaracetam intermediate
A technology of intermediates and compounds, applied in the field of preparation of buvaracetam intermediate-4-propylpyrrolidin-2-one, which can solve the problems of difficult industrial production and low chiral purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
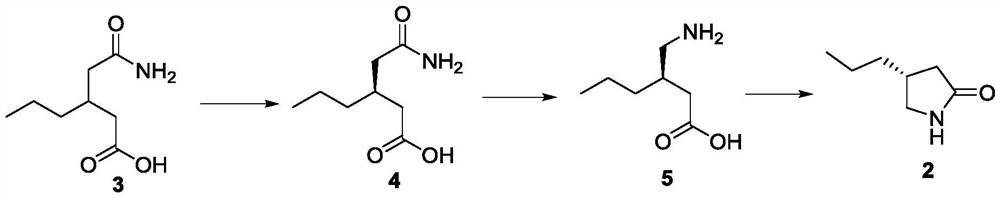
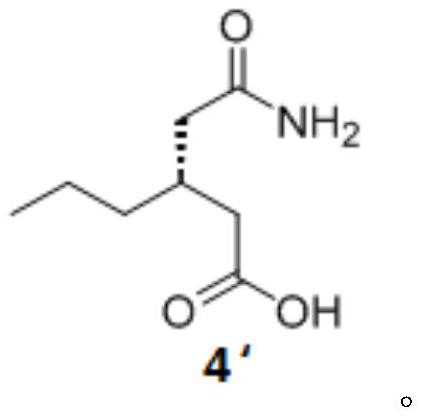
[0055] The preparation of embodiment 1 formula 4 compound
[0056] Add 4.5kg of chloroform to a 5L reactor, add 264g (1.524mol) of the compound of formula 3, heat to 45°C, add 153g (1.263mol) of S-phenylethylamine dropwise, and stir at 45°C for 10min, 5h after the dropwise addition Gradually lower the temperature to 25-30°C, keep stirring for 2 hours, filter, and rinse the filter cake with 1L chloroform once; transfer the filter cake to the reaction kettle, add a mixed solution of 4.5kg chloroform and 13g isopropanol, and stir at 25-30°C for 3h , filtered, the filter cake was rinsed once with 1 L of chloroform, and the filter cake was dried at 45°C. The two filter cakes are salts formed by the compound of formula 4 and S-phenethylamine, and a small amount of isomers, and the isomers in the first filter cake are less than the first filter cake.
[0057] Transfer the dried solid to a 500mL reaction flask, add 320g of water, cool down to -5-5°C, add 71.6g of concentrated hydroch...
Embodiment 2
[0059] The preparation of embodiment 2 formula 4 compounds
[0060] Add 4.5kg of chloroform to a 5L reaction kettle, add 264g (1.524mol) of the compound of formula 3, heat to 45°C, add 92.2g (0.762mol) of S-phenylethylamine dropwise, and stir at 45°C for 10min after the dropwise addition. Gradually lower the temperature to 25-30°C within 5 hours, keep stirring for 2 hours, filter, and rinse the filter cake with 1L chloroform once; transfer the filter cake to the reaction kettle, add a mixed solution of 4.5kg chloroform and 13g isopropanol, and stir at 25-30°C After 3 hours, filter, rinse the filter cake once with 1 L of chloroform, and dry the filter cake at 45°C.
[0061] Transfer the dried solid to a 500mL reaction flask, add 320g of water, cool down to -5-5°C, add 43.1g of concentrated hydrochloric acid dropwise while keeping warm, stir at -5-0°C for 1 hour, filter, and rinse the filter cake with 50mL of ice water , and dried the filter cake to obtain 111.1 g of white soli...
Embodiment 3
[0062] The preparation of embodiment 3 formula 4 compounds
[0063] Add 4.5kg of chloroform to a 5L reaction kettle, add 264g (1.524mol) of the compound of formula 3, heat to 45°C, add 184.4g (1.524mol) of S-phenylethylamine dropwise, and stir at 45°C for 10min after the dropwise addition. Gradually lower the temperature to 25-30°C within 5 hours, keep stirring for 2 hours, filter, and rinse the filter cake with 1L chloroform once; transfer the filter cake to the reaction kettle, add a mixed solution of 4.5kg chloroform and 13g isopropanol, and stir at 25-30°C After 3 hours, filter, rinse the filter cake once with 1 L of chloroform, and dry the filter cake at 45°C.
[0064] Transfer the above solid to a 500mL reaction flask, add 320g of water, cool down to -5-5°C, add 86.2g of concentrated hydrochloric acid dropwise while keeping warm, stir at -5-0°C for 1h, filter, rinse the filter cake with 50mL of ice water, and dry The filter cake was dried to obtain 110.1 g of white soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com