Recombinant antigen for detecting schistosoma japonicum katsurada as well as preparation method and application of recombinant antigen
A technology for recombinant antigens and schistosomes, applied in the biological field, can solve problems such as low sensitivity, insufficient sensitivity, and long detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
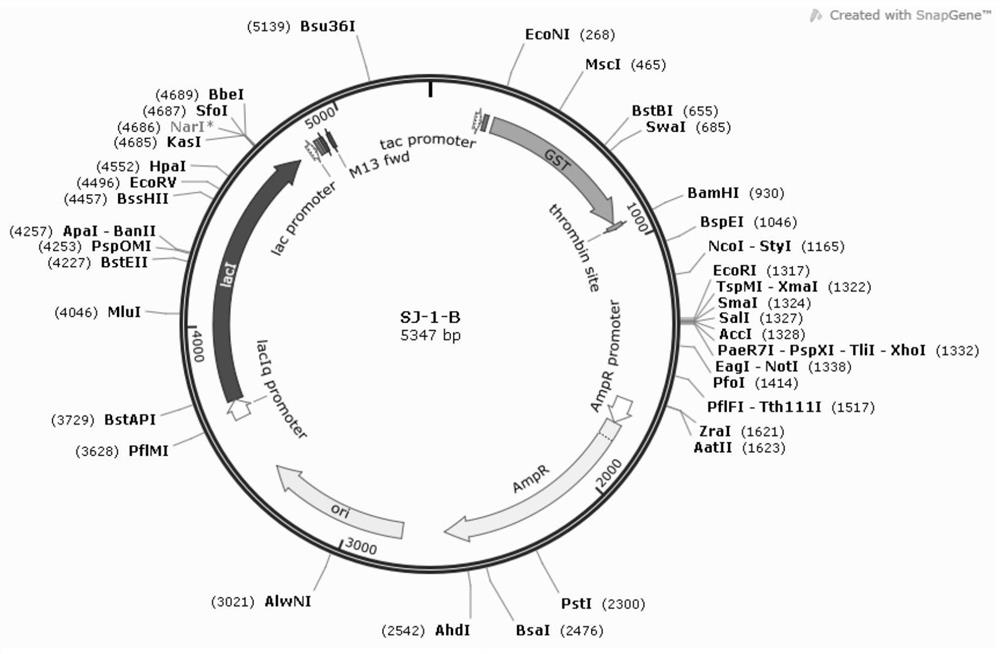
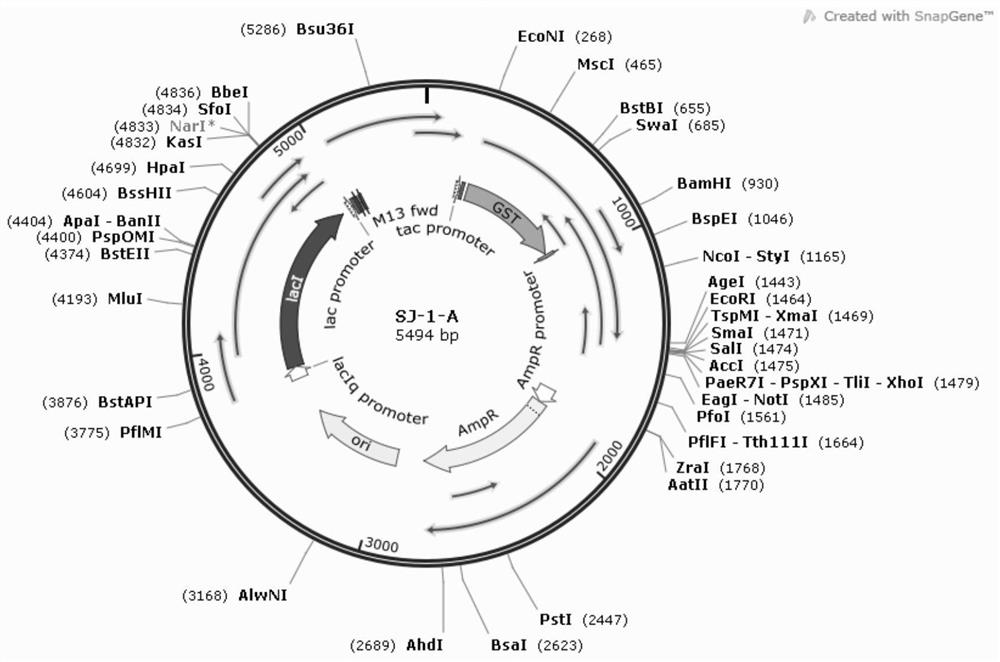
Image
Examples
Embodiment 1
[0027] Recombinant antigen 1 comprises GST protein fragment (SEQ ID NO.1), rSjGCP protein fragment (SEQ ID NO.2) and Sj23 (SEQ ID NO.3) protein fragment of Schistosoma japonicum connected sequentially from N-terminal to C-terminal, said The GST protein fragment and the rSjGCP protein fragment are connected through the connecting peptide 1 (SEQ ID NO.4: SDLEVLFQGPRGS) with the amino acid sequence shown in SEQ ID NO.4, and the rSjGCP protein fragment and the Sj23 protein fragment are connected through the amino acid sequence shown in SEQ ID NO. The connecting peptide 2 (SEQ ID NO.5: GGGGSGGGGS) shown in .5 is connected, and its amino acid sequence is shown in SEQ ID NO: 6:
[0028] MSPILGYWKIKGLVQPTRLLLEYLEEKYEEHLYERDEGDKWRNKKFELGLEFPNLPYYIDGDVKLTQSMAIIRYIADKHNMLGGCPKERAEISMLEGAVLDIRYGVSRIAYSKDFETLKVDFLSKLPEMLKMFEDRLCHKTYLNGDHVTHPDFMLYDALDVVLYMDPMCLDAFPKLVCFKKRIEAIPQIDKYLKSSKYIAWPLQGWQATFGGGDHPPKSDLEVLFQGPRGSMRIGYEGLPRDQWPKVIHWNLHARDGIIWVLDGLLKCPEKLCPLLAEDVDYYGGGGSGGGGSMTGALENPNE...
Embodiment 2
[0036] 50 rabbits were taken, infected with 300 Schistosoma japonicum, and then treated with praziquantel (80 mg / kg body weight) 6 weeks after infection. Blood samples were collected before infection, 2 weeks, 4 weeks, and 6 weeks after infection, and 2 weeks, 4 weeks, and 6 weeks after praziquantel treatment. In addition, the serum of 20 rabbits infected with Fasciola gigantea was collected by the same method as a reference substance, and the serum of 20 rabbits not infected was collected as a negative control substance. Add 100 ul of PBS (PH=7.6) containing recombinant antigen 1 shown in SEQ ID NO.7, recombinant antigen 2 or GST protein shown in SEQ ID NO.8 at a concentration of 20 ug / ml in a polystyrene 96 microwell plate Coating was performed overnight at 4°C, and then rabbit serum diluted 1:100 was added to each well and incubated at 37°C for 1 hour. Add goat anti-mouse IgG-coupled horseradish peroxidase antibody and incubate at 37°C for 3 hours, then add 200ul of PBS co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com