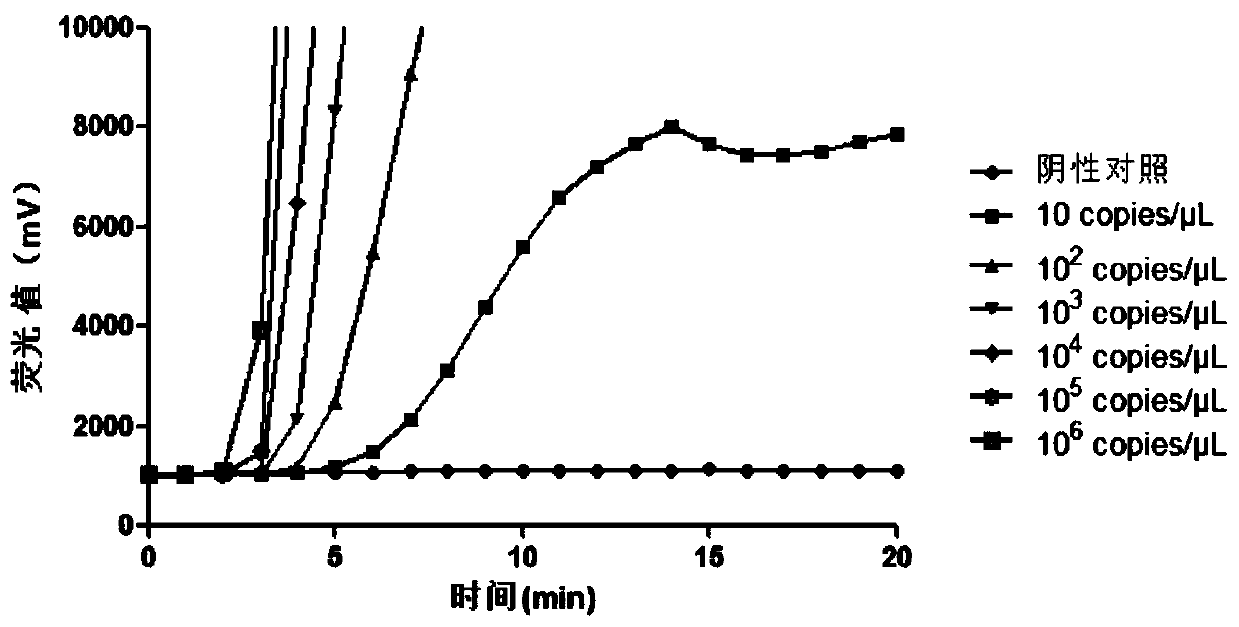
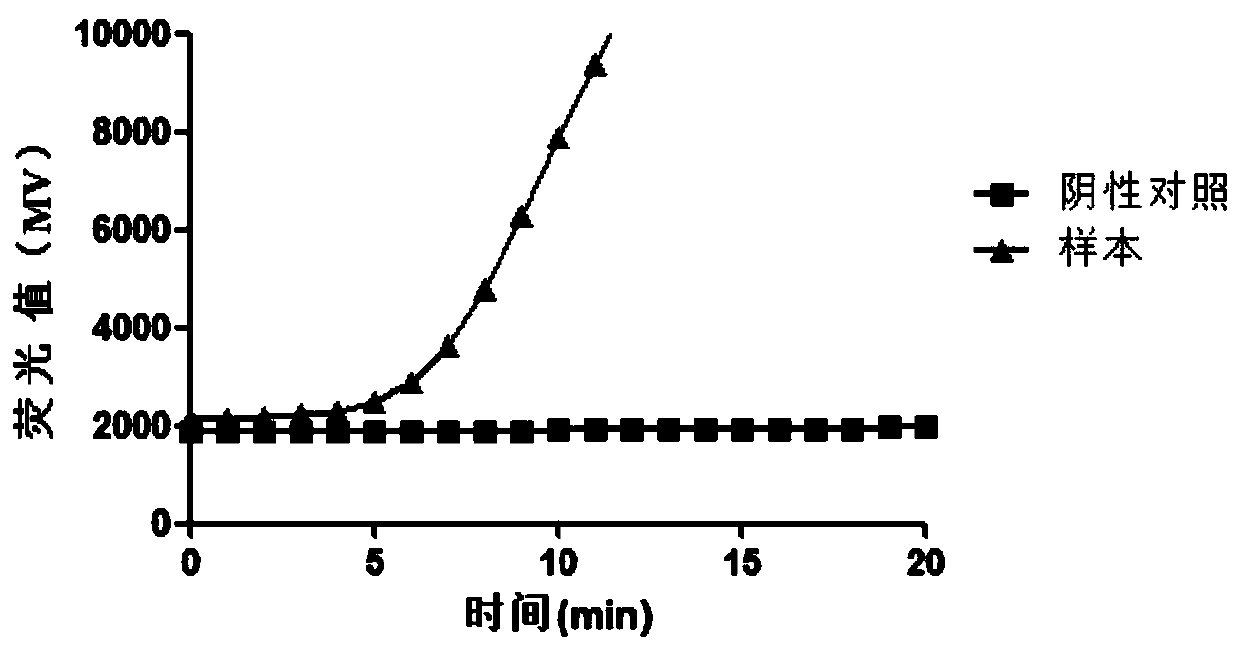
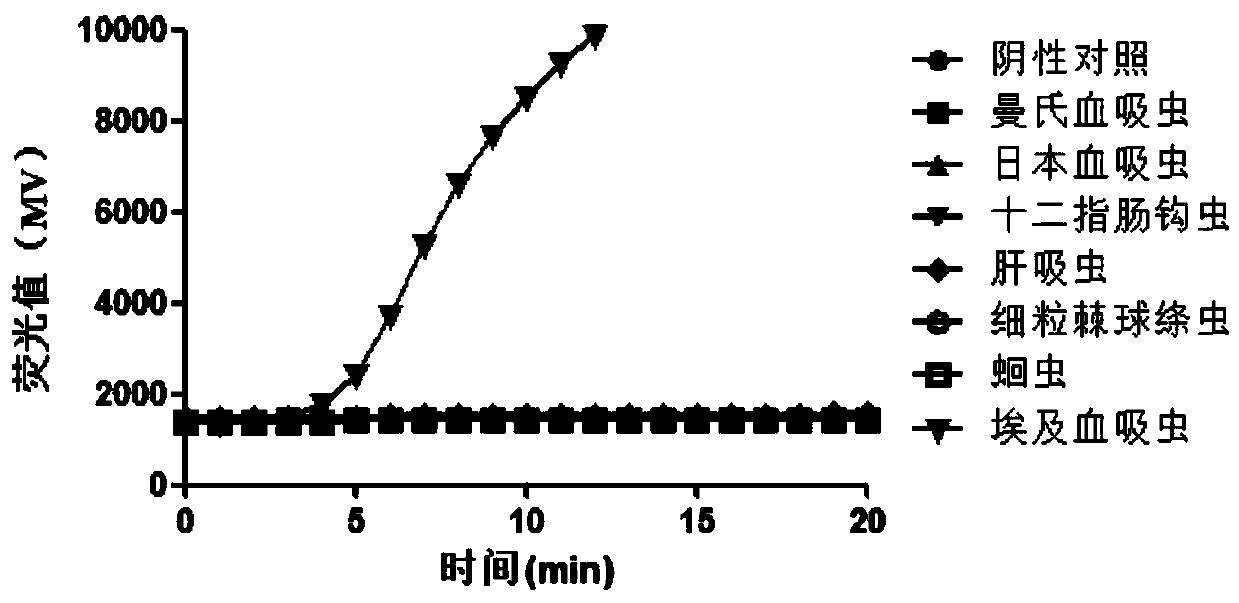
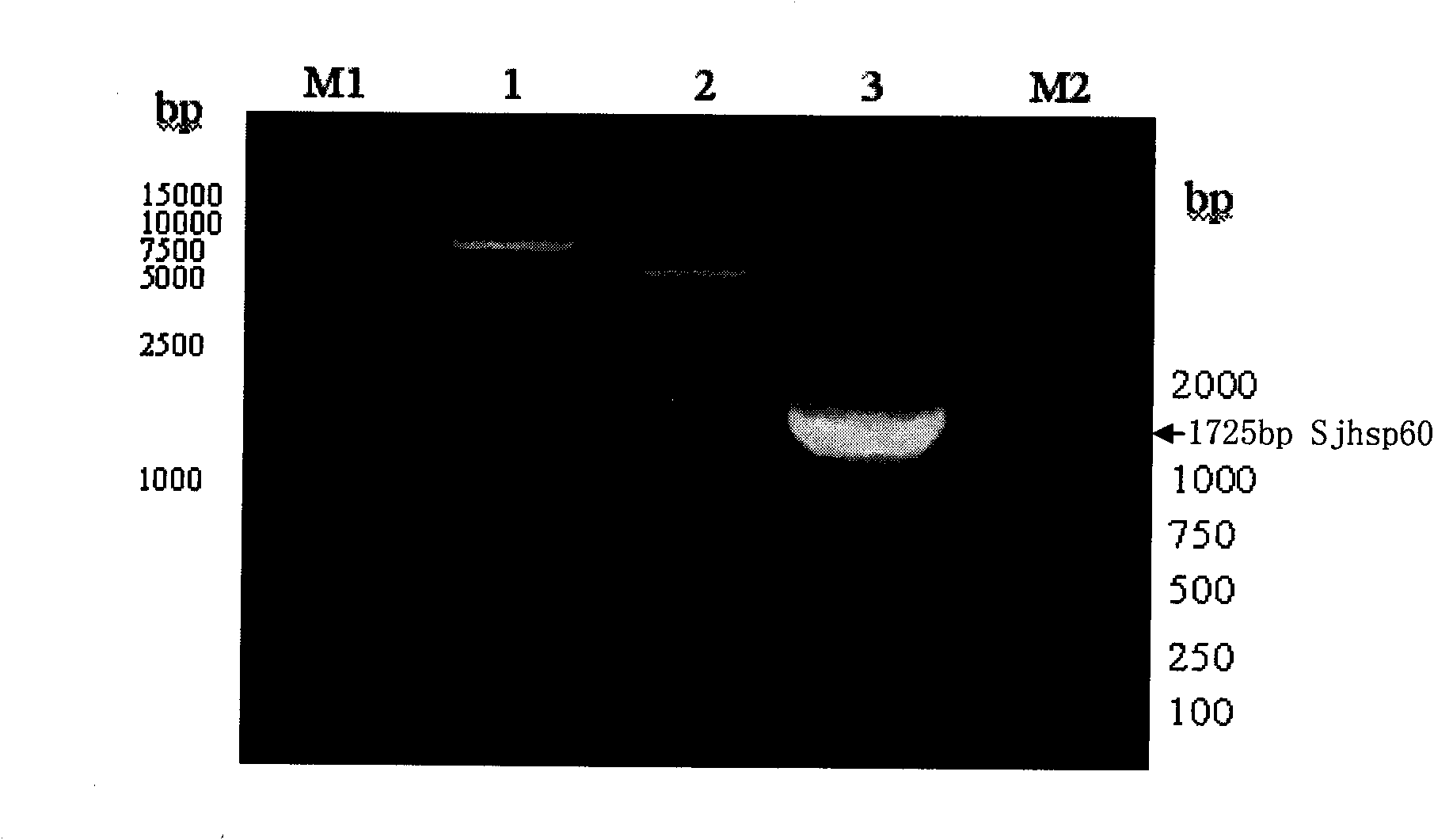
Patents
Literature
65 results about "Schistosoma sinensium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof
ActiveCN105384803AImproving immunogenicityGood antigenicityBiological material analysisAntiparasitic agentsDIAGNOSTIC ANTIGENSFhit gene
The invention provides a schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof. The protein has an amino acid sequence shown as SEQ ID NO.2 or an amino acid sequence having a same function, formed by replacing, omitting and / or adding one or more amino acid residues for the amino acid sequence shown as SEQ ID No.2. The invention also provides a gene sequence for encoding the protein. The recombinant protein SjSAPLP4 is good in immunogenicity, can be used as an excellent diagnostic antigen, can be used for preparing a schistosoma japonicum katsurada diagnosis kit having high sensitivity and high specificity, also can be used for preparing an anti-schistosome vaccine and can be used as a potential drug acting target spot to screen the drug for treating the schistosoma japonicum katsurada.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
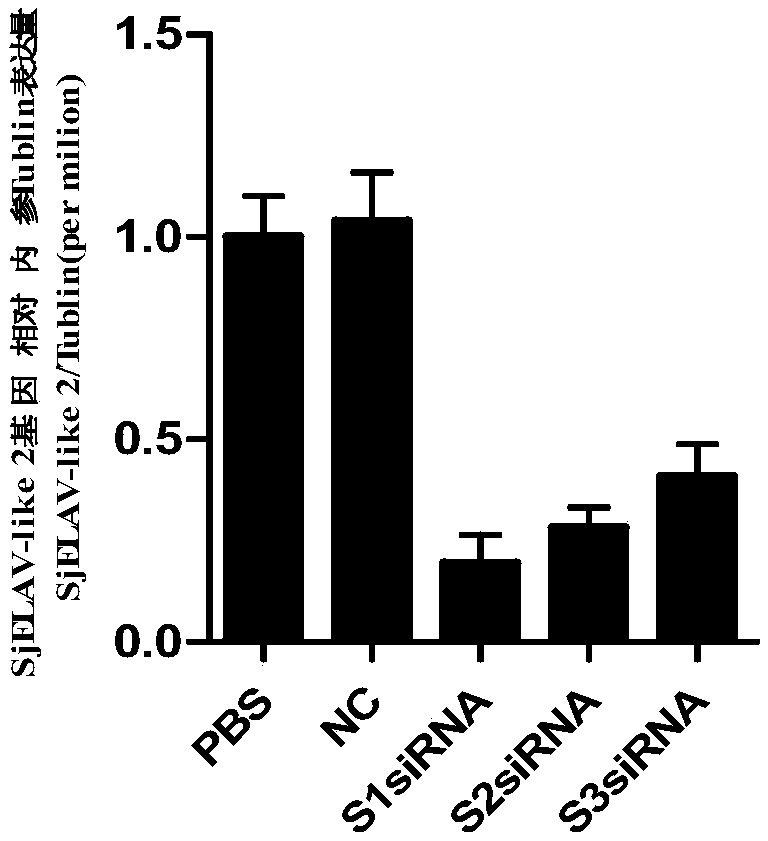
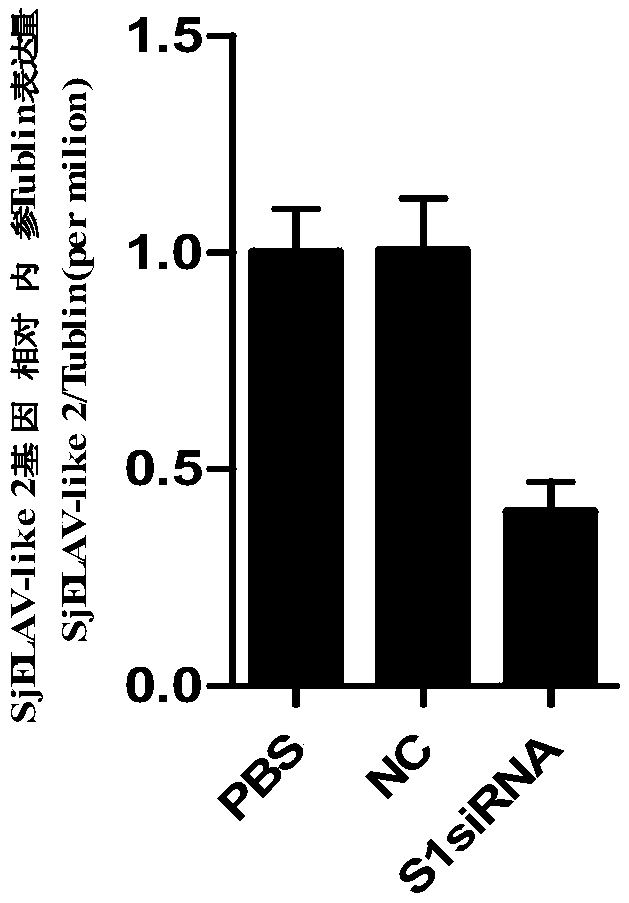
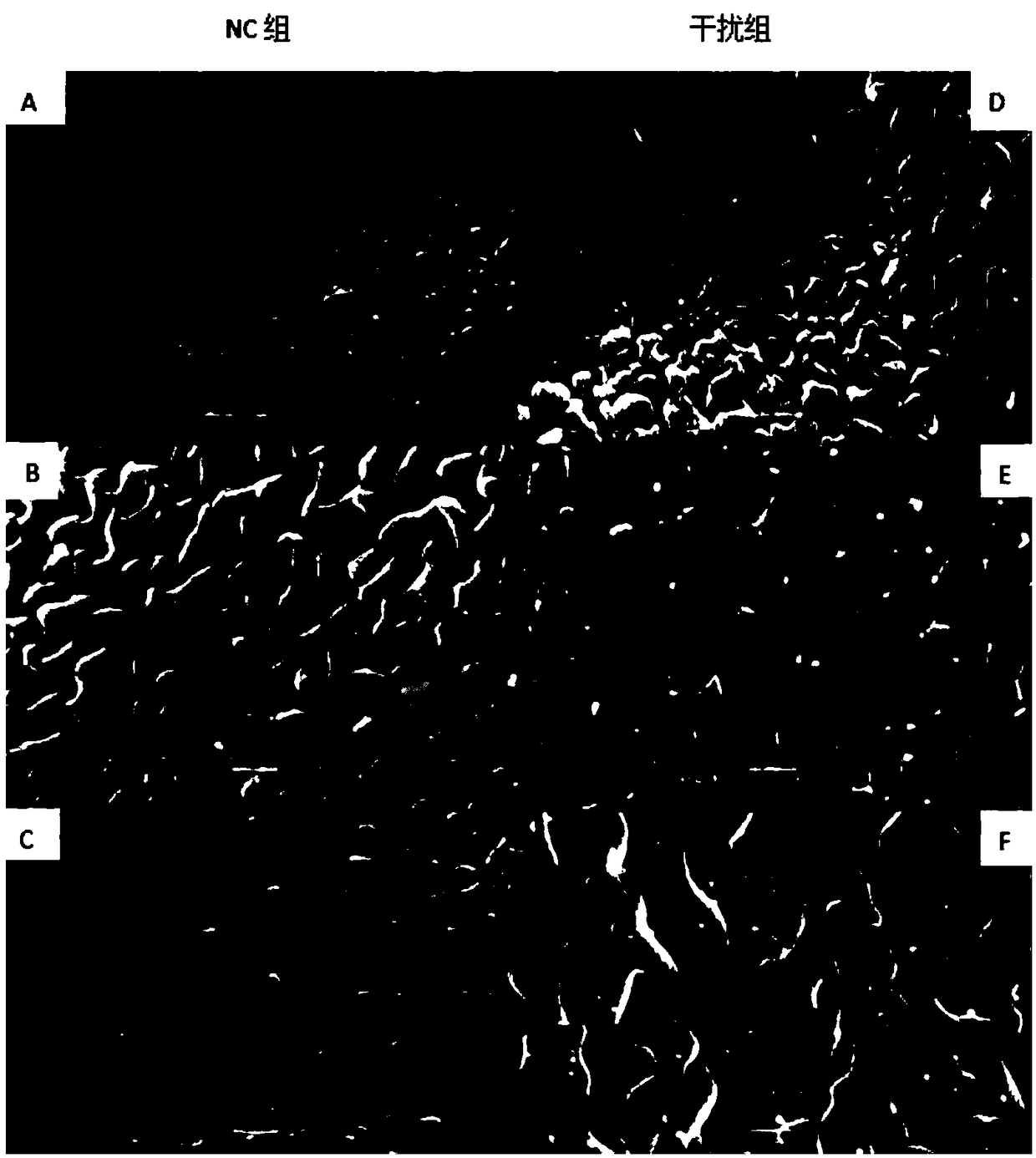
siRNA of schistosoma japonicum SjELAV-like 2 genes and application thereof
ActiveCN108795934ALower hatch rateSuitable for preparationOrganic active ingredientsAntiparasitic agentsDrugBlood fluke infection
The invention discloses siRNA of schistosoma japonicum SjELAV-like 2 genes. The siRNA is selected from one pair of a combination of more than optional two pairs as follows: nucleotide sequences shownas SEQ ID NO.1 and SEQ ID NO.2, nucleotide sequences shown as SEQ ID NO.3 and SEQ ID NO.4, and nucleotide sequences shown as SEQ ID NO.5 and SEQ ID NO.6. The invention further discloses the application of the siRNA of schistosoma japonicum SjELAV-like 2 genes. The siRNA of schistosoma japonicum SjELAV-like 2 genes disclosed by the invention is capable of obviously inhibiting transcription of the schistosoma japonicum SjELAV-like 2 genes and obviously reducing the liver egg hatching rate of schistosomiasis infected mice, and is applicable to preparation of drugs for treating schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Primers, probe, kit and method for RPA-LFD visualization rapid detection of Schistosoma nucleic acid
PendingCN111778344AIncreased sensitivityNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationSchistosomiasesSchistosoma haematobium
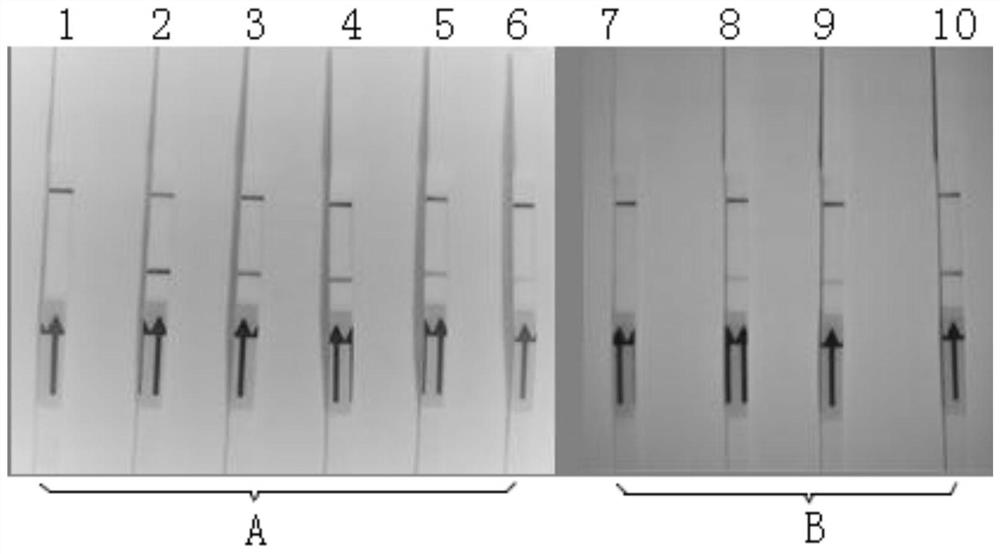
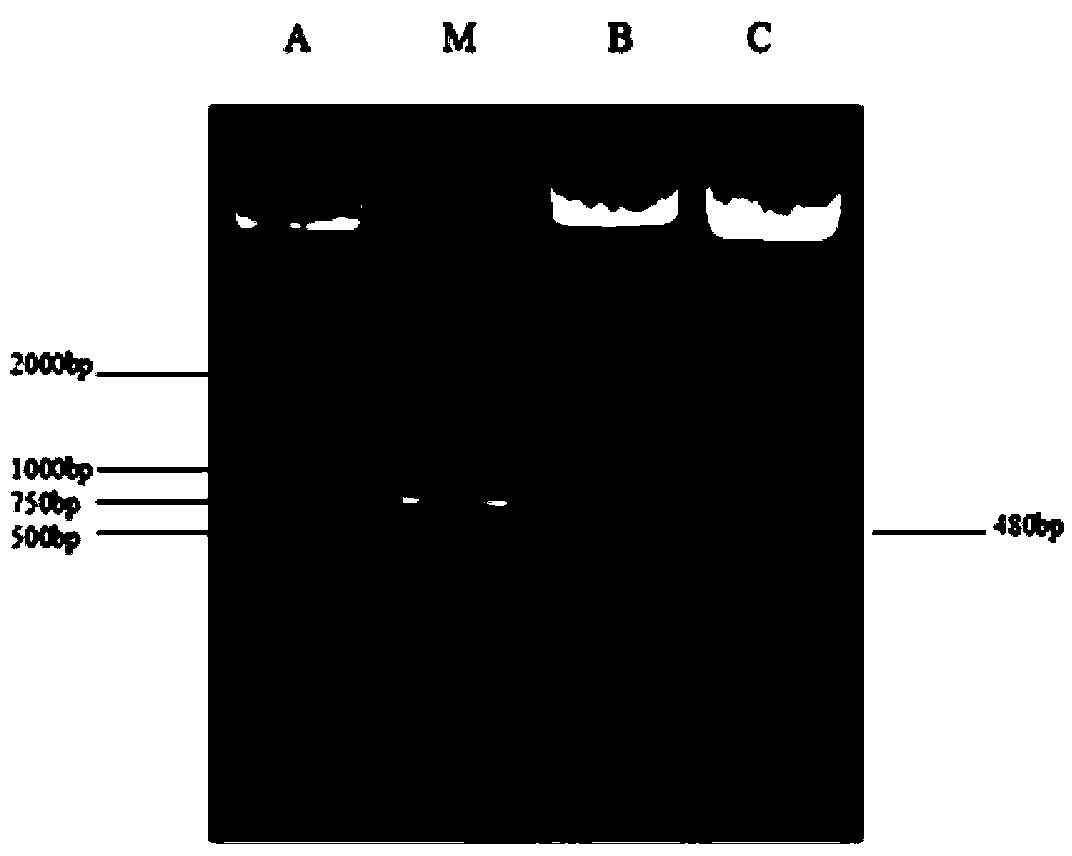
The invention discloses primers, probe, kit and method for the RPA-LFD visualization rapid detection of Schistosoma nucleic acid. Sequences of the primers are as shown in SEQ ID NO.1-2, and a sequenceof the probe is as shown in SEQ ID NO.3. The RPA primers and probe are designed with the SjCHGCS19 gene as a target sequence, and the sensitive and rapid visualization detection of the schistosome nucleic acid is realized by using a RPA amplification technology combined with lateral flow chromatography test strip method. The detection method of the invention has a detection limit of 1 fg for Schistosoma japonicum genome DNA, and is expected to be used in the general detection of Schistosoma mansoni and Schistosoma haematobium. The method can detect circulating nucleic acid of Schistosoma in the serum of mice at an early stage of infection, the operation is simple and fast, no special equipment is required, a reaction temperature is close to room temperature, results can be observed with the naked eye, and the realization of the early and sensitive and rapid detection of intermediate hosts of Schistosoma on site, and the timely and sensitive monitoring of environments with high schistosomiasis transmission risks are facilitated.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心 +1
Method and kit for detection of schistosomiasis through polymerase chain reaction
The present invention provides a method of detection of Schistosoma sp. in biological samples through PCR, as well as a method for the diagnosis of Schistosomiasis through the amplification by PCR of the DNA sequence of Schistosoma sp., followed by the separation of the products of the amplification by electrophoresis and the detection by appropriate technique. Moreover, the present invention provides a kit for the diagnosis to be used in the detection of Schistomsomiasis.
Owner:奥斯瓦尔多克鲁兹基金会
Schistosoma japonicum katsurada recombinant antigen as well as preparation method and application thereof
The invention discloses schistosoma japonicum katsurada recombinant antigen. The recombinant antigen comprises an amino acid sequence of schistosoma japonicum katsurada PGMRC2 protein indicated by SEQ ID No.1. The invention also discloses a preparation method of schistosoma japonicum katsurada antigen and application of the antigen in preparing a vaccine or a medicament for preventing or treating the schistosoma japonicum katsurada disease. By adopting the schistosoma japonicum katsurada recombinant antigen, the specificity IgG, IgG1 and IgG1a antibodies for resisting the recombinant antigen can be induced in a mice body, the worm reduction rates of 22.08 percent and 20.097 percent are respectively induced in two animal protection experiments, so that the recombinant antigen is suitable for being used as a candidate vaccine for resisting the schistosoma japonicum katsurada and has good application prospect.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Schistosoma japonicum calcium-binding EF-hand domain containing protein (SjEFCAB) recombinant antigen protein and preparation method and application thereof
InactiveCN103374567AIncreased sensitivityImprove featuresBiological testingAnimals/human peptidesProtein CTarget antigen
The invention discloses a preparation method of a Schistosoma japonicum calcium-binding EF-hand domain containing protein (SjEFCAB) recombinant antigen protein. The preparation method comprises the following steps of: designing SEQ ID NO.3 or a complementary chain 5' thereof as a primer and SEQ ID NO.4 or a complementary chain 3' thereof as a primer, and carrying out amplification on a Schistosoma japonicum SjEFCAB gene sequence; establishing and identifying Schistosoma japonicum SjEFCAB recombinant plasmids; and carrying out induction expression, purification and the like on recombinant protein. Besides, the invention further discloses an application of the Schistosoma japonicum SjEFCAB recombinant antigen protein prepared by using the method in preparation of products for detecting a serum antibody of a Schistosoma japonicum patient. Enzyme linked immunosorbent assay (ELISA) proves that the Schistosoma japonicum SjEFCAB recombinant antigen protein has higher sensitivity and specificity when used for diagnosing the Schistosoma japonicum, is a potential candidate target of diagnosis, and can be used as a target antigen for diagnosing the Schistosoma japonicum.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Schistosoma japonicum katsurada recombinant protein SjSAPLP5 as well as encoding gene and application thereof
ActiveCN105254732AImproving immunogenicityGood antigenicityBiological material analysisAntiparasitic agentsAntigenDisease
The invention provides a schistosoma japonicum katsurada recombinant protein SjSAPLP5 as well as an encoding gene and an application thereof. The protein provided by the invention has amino acid sequence as shown in SEQ ID No.2 or has amino acid sequence which is formed by replacement, deletion and / or addition of one or more of amino acid residues and has the same function. The invention also provides the encoding gene for encoding the protein. The recombinant protein SjSAPLP5 provided by the invention is excellent in immunogenicity, can be served as an excellent diagnosis antigen, is used for preparing a schistosoma japonicum katsurada diagnostic kit with high sensitivity and high specificity, and is also used for preparing an anti-schistosome vaccine and the drug served as a potential drug effecting target spot and is used for screening and treating the schistosoma japonicum katsurada diseases.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
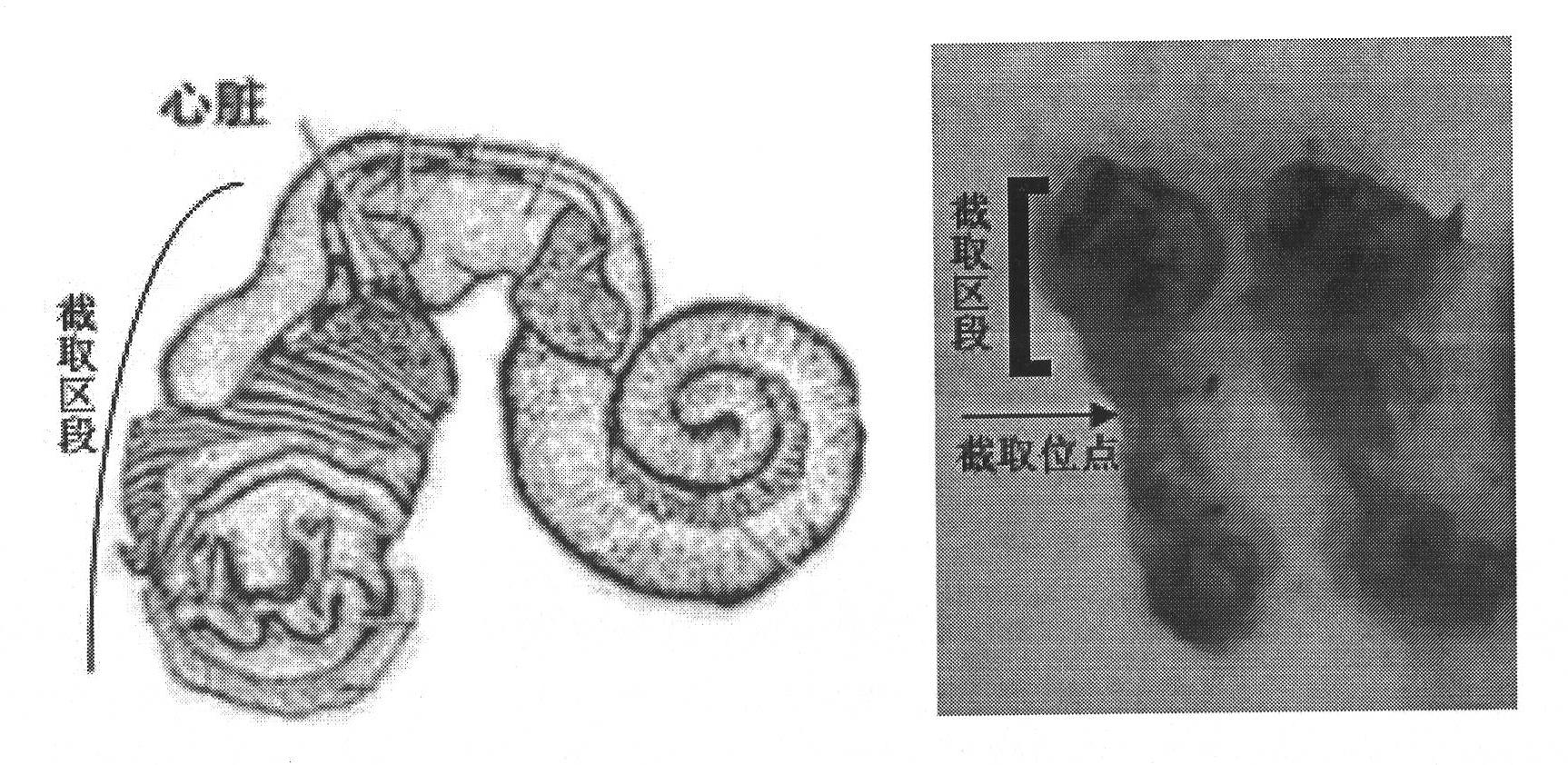
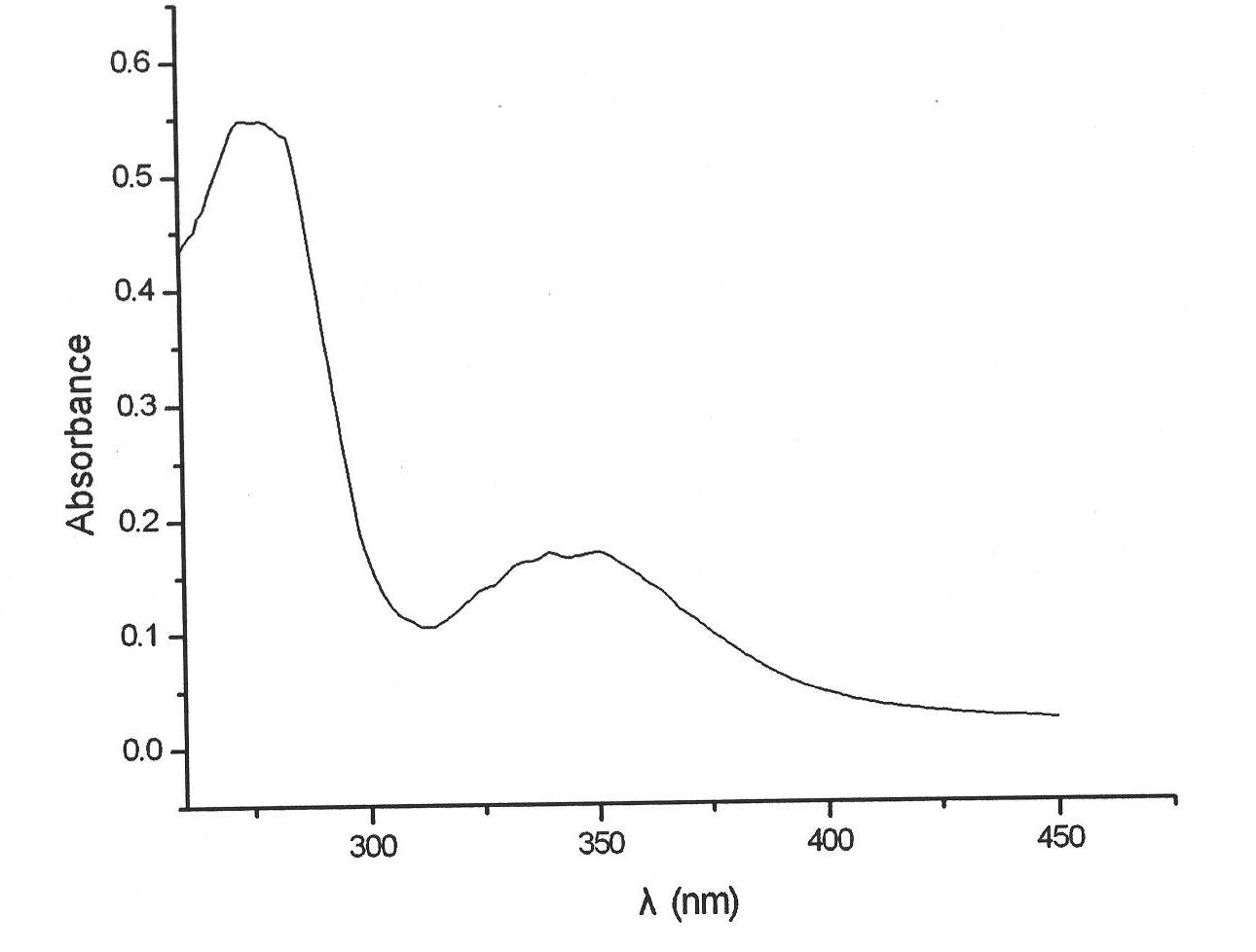
Preparation method of snail respiratory protein
The present invention relates to a preparation method of a snail respiratory protein. A respiratory protein containing copper ions is extracted from a snail vivo used as an intermediate host of Schistosoma japonicum by virtue of mechanical extrusion, tissue dissection, hemolymph extraction, ammonium sulfate precipitation, super high-speed centrifugal separation and other means. The respiratory protein contains 0.2-0.3% of copper, the UV characteristic absorption peak is at 275nm and 340nm, and a cylindrical agreegate structure is observed under a transmission electron microscope.
Owner:GANNAN NORMAL UNIV
Method for quickly separating Schistosoma japonicum immature egg
InactiveCN101055234AImprove work efficiencyHelp removeAnimal cellsPreparing sample for investigationAntigenDrug biological activity
The invention discloses a method of fast separating Japan schistosome unfledged spawn, which employs technique of combining repeat sieving and pancreatin processing and completes in two hours. Purity of the obtained spawn is above 99%, structure of the spawn is integrated and good biological activity is remained, which can satisfy basic need of antigen extraction and analysis. Comparing to exist technique, yield of the spawn is largely increased.
Owner:汪世平 +1
Genes highly expressed in Schistosoma japonicum schistosomulum and encoded proteins and application thereof
ActiveCN110330556AHigh detection sensitivityStrong specificityBiological material analysisAntiparasitic agentsAntigenSerum ige
The present invention provides genes highly expressed in Schistosoma japonicum schistosomulum and encoded proteins and application thereof. A whole-genome expression profile chip of Schistosoma japonicum is utilized to screen a series of genes highly expressed in Schistosoma japonicum schistosomulum, and antigen proteins encoded by genes which are SjScP27, SjScP80, SjScP84 and SjScP88 can be specifically recognized by serum of patients with schistosomiasis and show obvious positive reactions. ELISA detection proves that the antigen proteins have high sensitivity and specificity for Schistosomajaponicum detection, and can be used for developing diagnostic reagents for schistosomiasis japonica.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Primer group, probe and kit for detecting schistosoma haematobium
PendingCN110923350ALow technical requirementsSimple and fast operationMicrobiological testing/measurementDNA/RNA fragmentationSchistosoma haematobiumSchistosoma sinensium
The invention provides a primer group for detecting schistosoma haematobium. The primer group comprises an upstream primer and a downstream primer, wherein the sequence of the upstream primer is shownas SEQ ID No. 1; and the sequence of the downstream primer is shown as SEQ ID No. 2. The primer group, probe and kit for detecting the schistosoma haematobium are simple and convenient to operate; tests prove that when the primer group, probe and kit for detecting the schistosoma haematobium are used for detecting the schistosoma haematobium, the specificity is high, the sensitivity and the accuracy are high, the repeatability is good, a detection result can be obtained within 20 minutes, the requirements on detection conditions and the technical requirements of detection personnel are low, and the detection cost is low.
Owner:JIANGSU INST OF PARASITIC DISEASES +1
Recombinant expression carrier containing schistosoma japonicum gene and application thereof
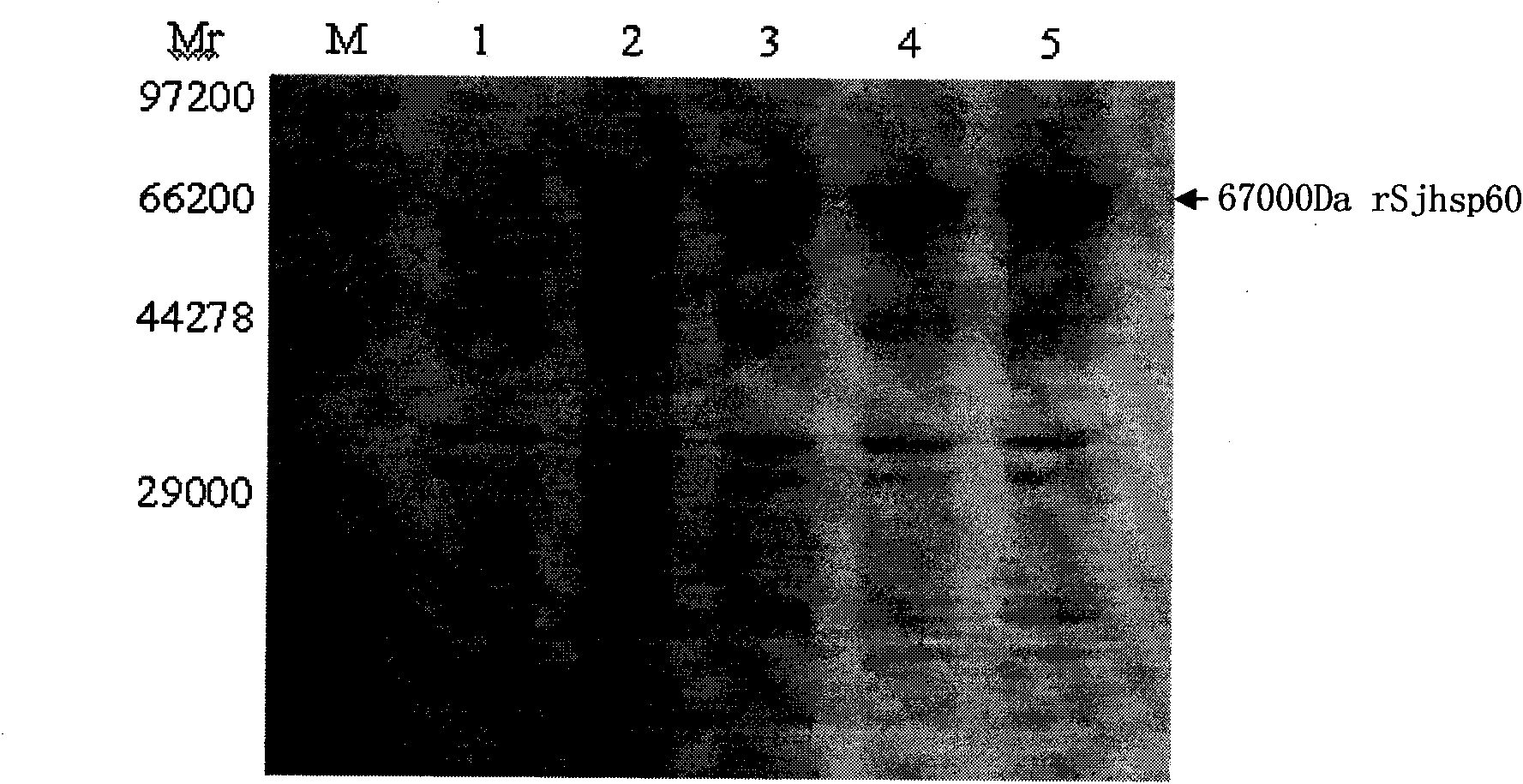
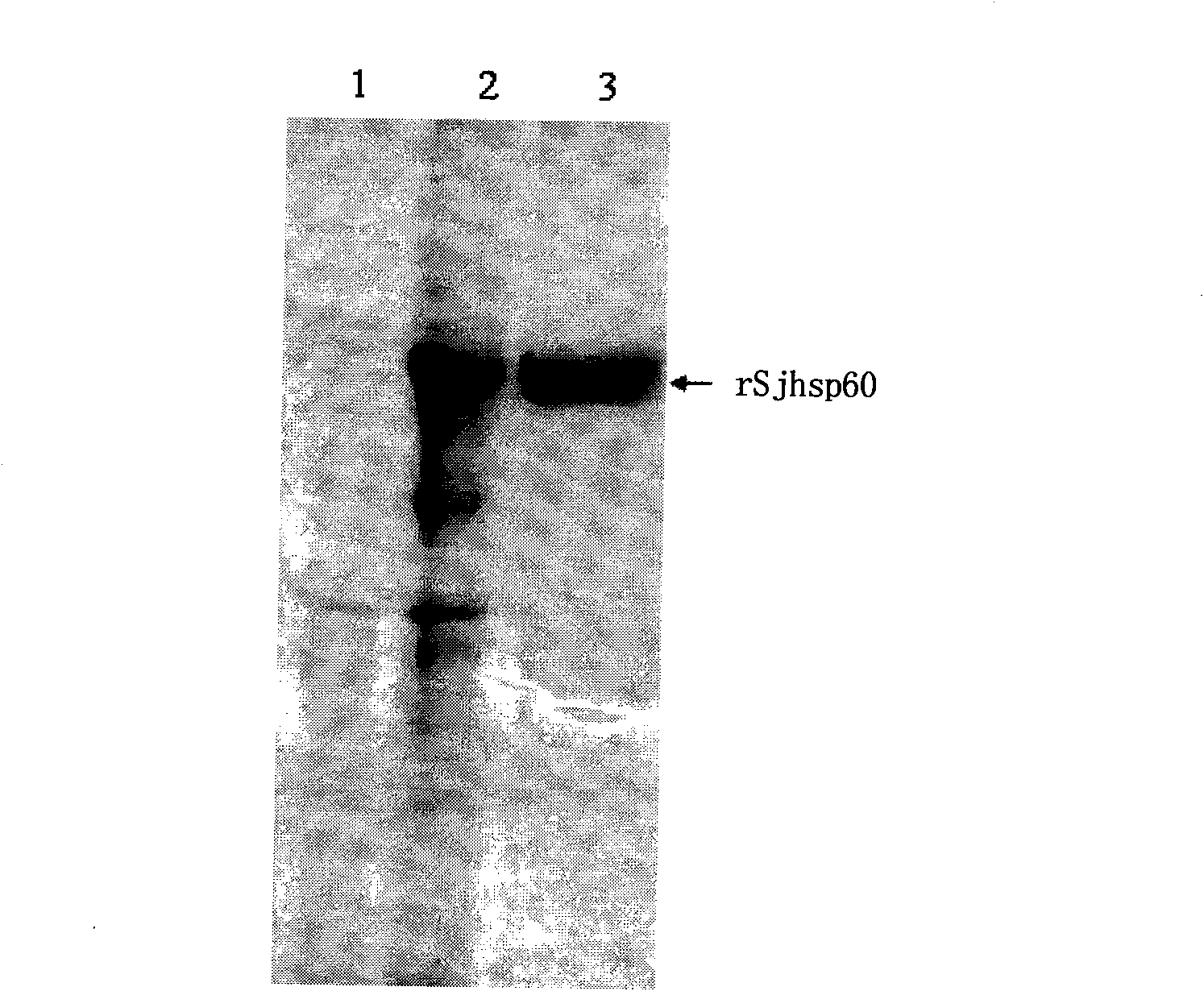
The invention discloses a recombinant expression carrier containing a schistosoma japonicum gene which is a schistosoma japonicum heat shock protein 60 gene. The invention also discloses a preparation method of the schistosoma japonicum heat shock protein 60 and the application of the recombinant expression carrier. The recombinant expression carrier containing the schistosoma japonicum gene can highly express the schistosoma japonicum heat shock protein 60 gene, and the highly expressed recombinant schistosoma japonicum Hsp60 protein can induce mouse to generate the protection function of resisting the infection of schistosoma japonicum at a certain level.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Primer, probe, kit and method for visually and rapidly detecting nucleic acid of schistosoma japonicum katsurada through LFD-RPA
PendingCN113025726AIncreased sensitivityHave recognition valueMicrobiological testing/measurementDNA/RNA fragmentationGenomeSchistosoma sinensium
The invention discloses a primer, a probe, a kit and a method for visually and rapidly detecting nucleic acid of schistosoma japonicum katsurada through LFD-RPA. The sequence of the primer is shown as SEQ ID NO.1-2, and the sequence of the probe is shown as SEQ ID NO.3. The RPA primer and the probe are designed by taking a schistosoma japonicum G55A (SjG55A) gene as a target sequence, the sensitive and rapid visual detection of schistosoma japonicum nucleic acid is realized by adopting an RPA amplification technology in combination with a lateral flow chromatography test strip method, the detection limit of schistosoma japonicum genome DNA can reach 10fg, and the identification detection of schistosoma japonicum and schistosoma mansoni is expected to be realized. One positive oncomelania in 1500 negative oncomelania can be detected, operation is simple and rapid, no special instrument is needed, the reaction temperature is close to the room temperature, the result can be observed by naked eyes, and detection and monitoring of the schistosome intermediate host in a laboratory and on site are facilitated.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Preparation of killing oncomelania from pachysand and its process and use
The present invention relates to the field of biological technology. The pachysand extract or steroid alkaloid produced through crushing and magnetic stirring extraction process is compounded into water solution of different concentration to kill Oncomelania hupensis as the host of Schistosoma japonicum. It has obvious killing effect and is harmless to human body and farm animal.
Owner:HUAZHONG NORMAL UNIV
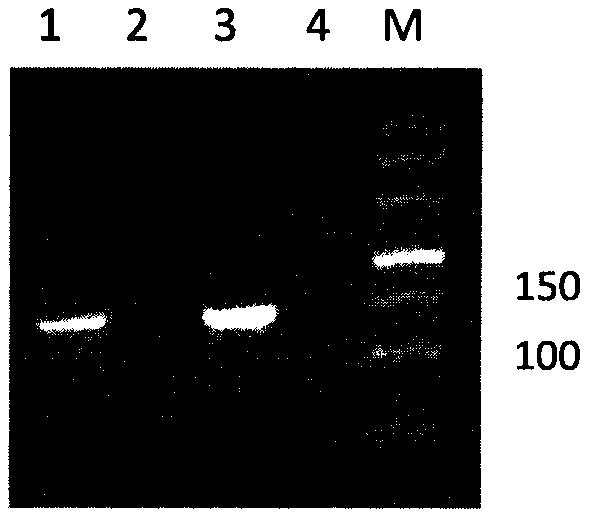
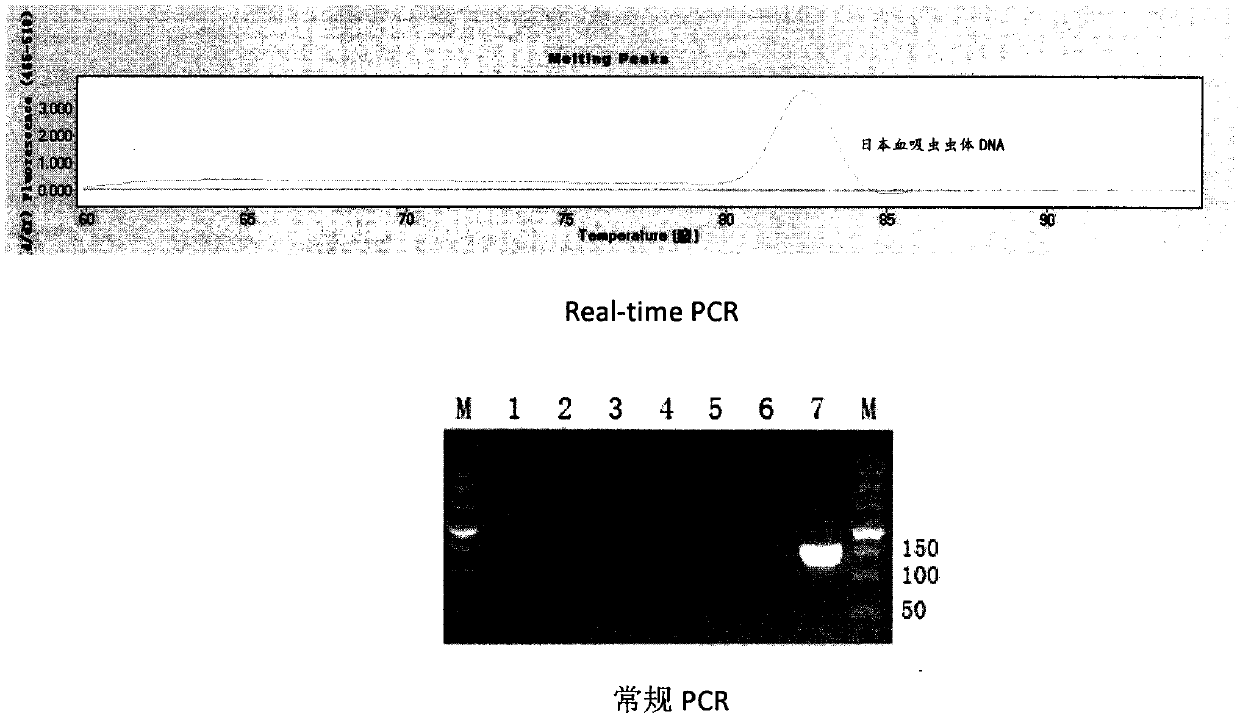
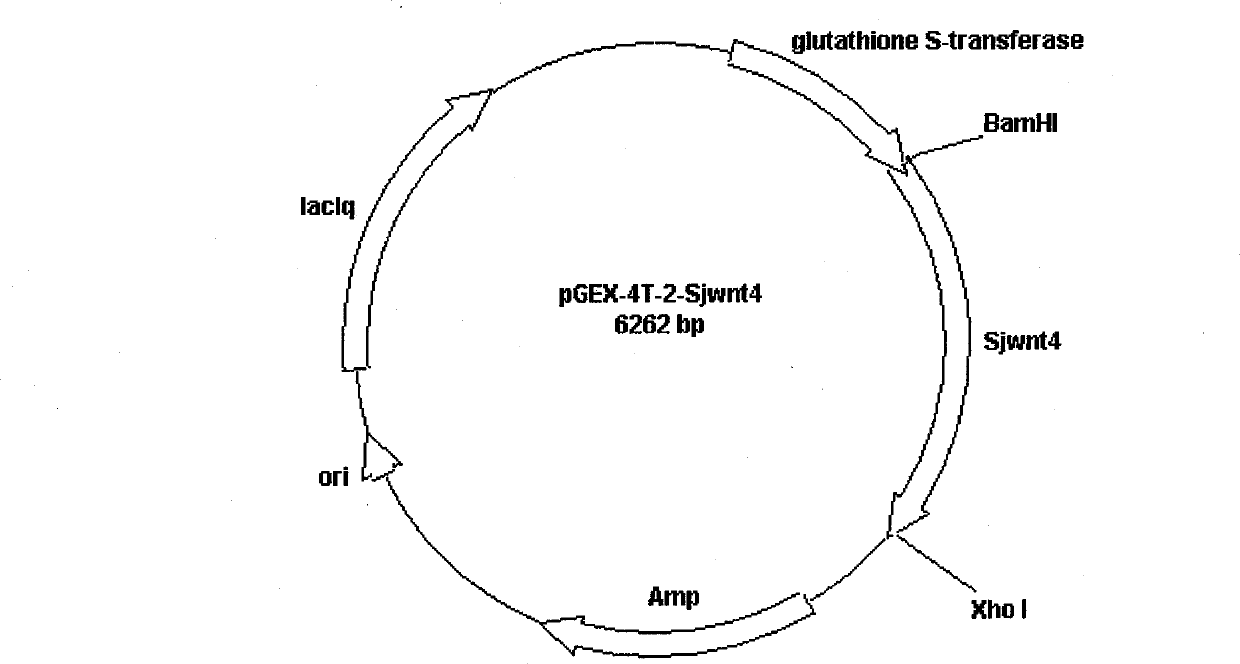
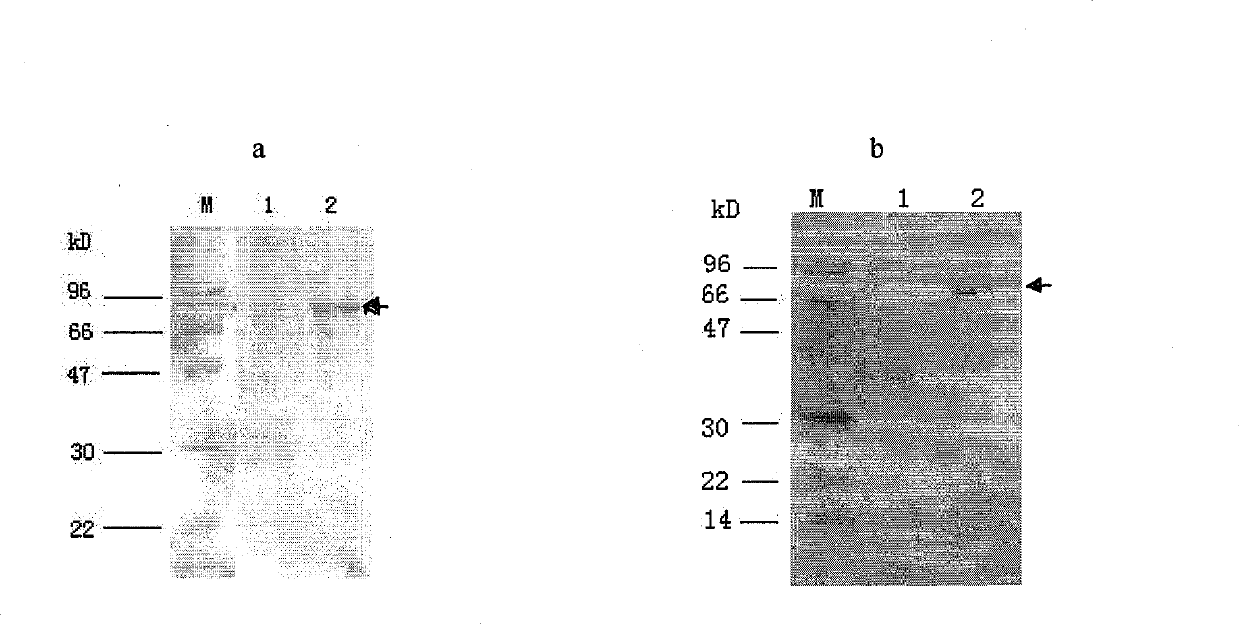
Clone, expression and use of Schistosoma Japonicum signal transduction protein Sjwnt-4 gene
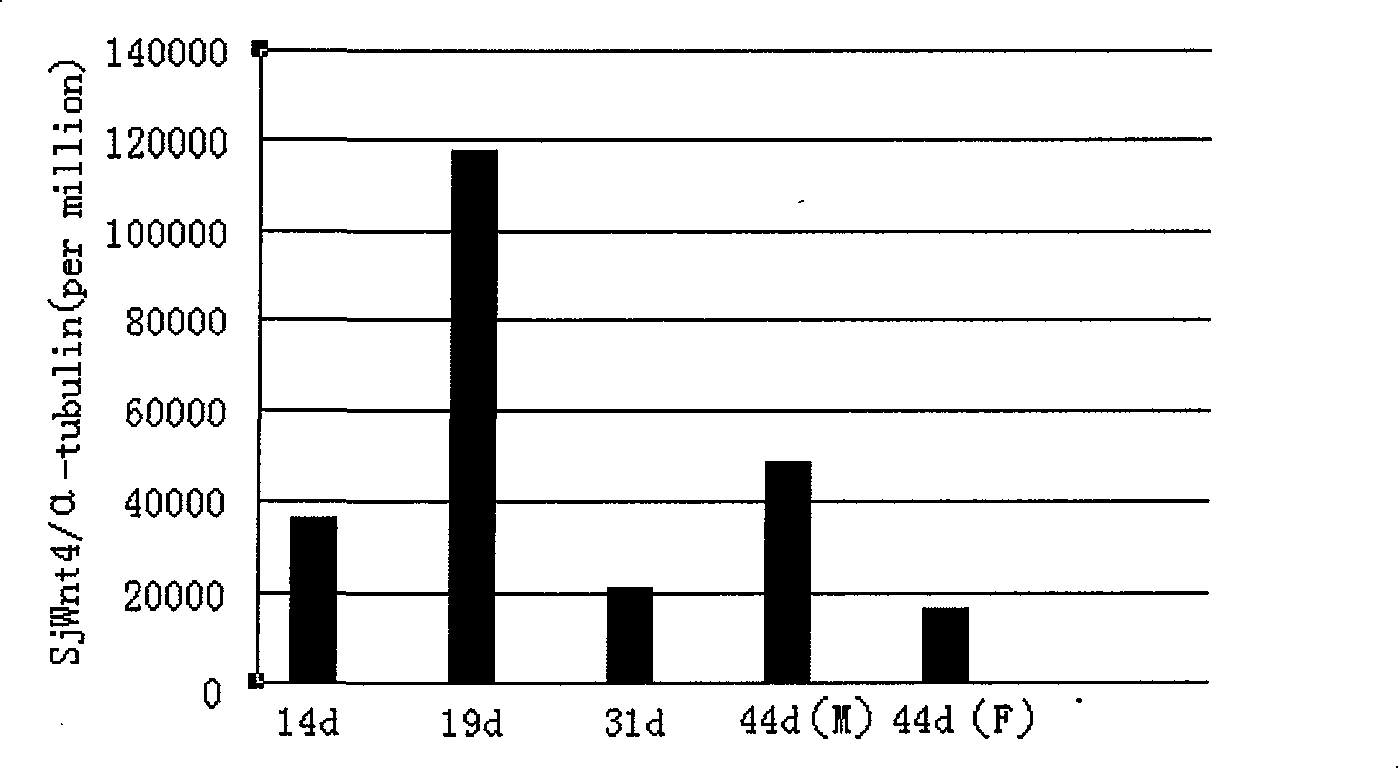
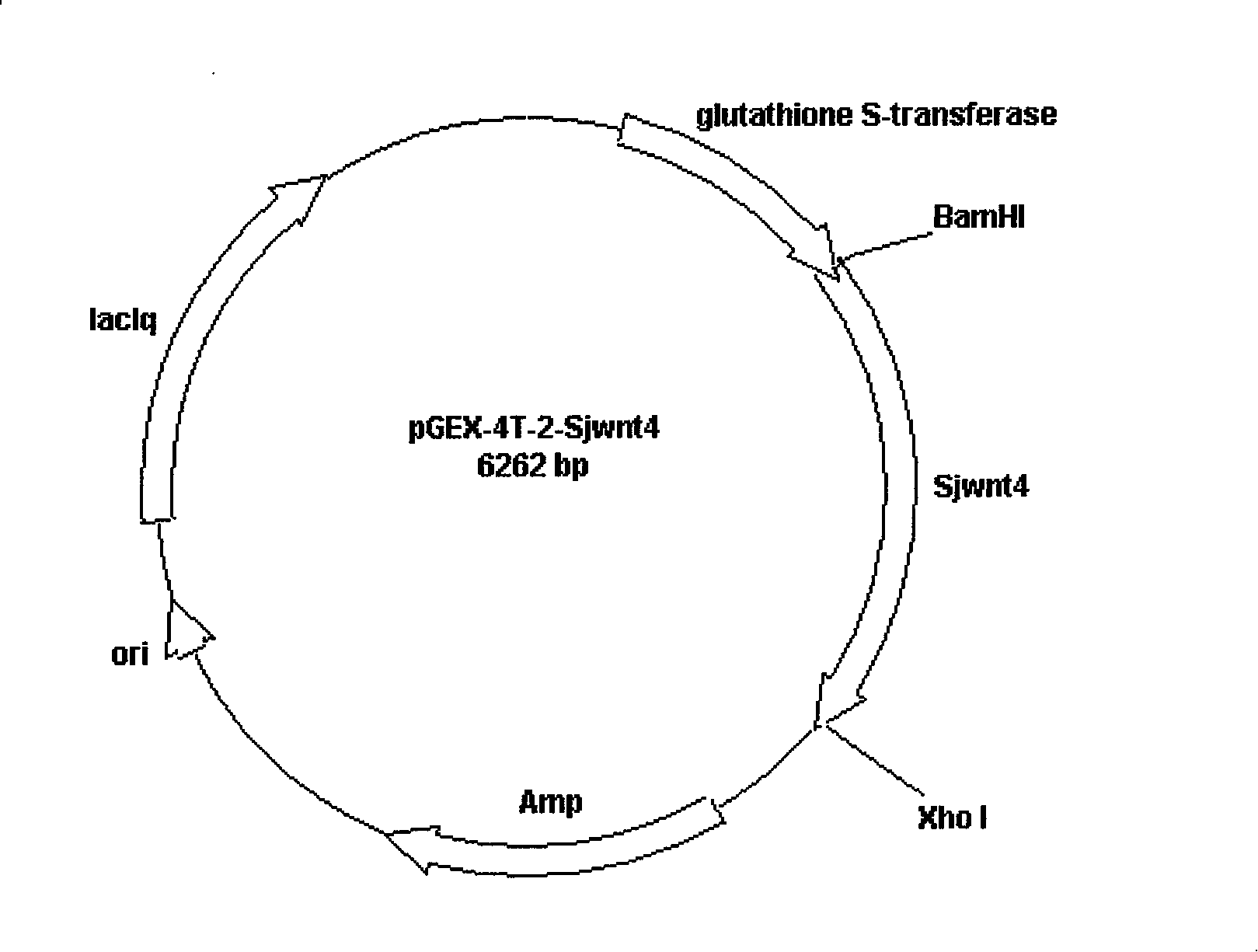
The invention discloses a Wnt family gene which is amplified for the first time from Schistosoma japonicum 19-day schistosomulum by utilization of RACE technology, wherein, sequence analysis indicates that: a complete coding frame of the gene comprises 1311bp, 436 amino acids coded; and theoretical molecular weight of 49.6kD. Homology analysis results indicate that: amino acid sequences of the gene have typical Wnt family protein characteristics; similarity of the amino acid sequences with amino acid sequences of Dugesia japonica and human Wnt4 is relatively 43 percent and 37 percent; the gene is presumed to be a Wnt4 gene of schistosome and named as Sjwnt4 (GenBanK Log-On No.: DQ643829). Real-time quantitative PCR analysis indicates that: the gene is expressed all in 14-day schistosomulum, 19-day schistosomulum, 31-day imago, 44-day female worms and 44-day male worms. The invention constructs a pronucleus expression vector pGEX-4T-2-Sjwnt4 of the gene and applies an Escherichia coli system for expression; expression proteins exist in the form of inclusion bodies; Western blottings indicate that expression products can be identified by crude antigen immune serums of Schistosoma japonicum imago.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Fusion gene of Japan schistosome antigen gene and constituted DNA vaccin and preparing process
InactiveCN1904053AImprove immunityEasy to produceProtozoa antigen ingredientsMicrobiological testing/measurementAntigenCancer research
The present invention discloses a fusion gene of schistosoma japonicum antigen genes, formed DNA vaccine and its preparation method. Said schistosoma japonicum DNA polyvalent vaccine is composed of schistosoma japonicum antigen genes (including sj23, sj FABP, sj26, sj GAPDSH, sj TPI and sj 97) and fusion gene of schistosoma japonicum antigen genes and eukaryotic expression vector with several insertion sites. The fusion gene of 30 schistosoma japonicum antigen genes is obtained by using 6 schistosoma japonicum antigen genes through 'fusion PCR' preparation process. Said fusion gene or antigen gene can be inserted into eukaryotic expression vector so as to obtain the invented schistosoma japonicum DNA polyvalent vaccine.
Owner:HUAZHONG UNIV OF SCI & TECH
Nucleic acid diagnosis kit for detecting schistosoma japonicum and detection method with nucleic acid diagnosis kit
InactiveCN110760588AIncreased sensitivityAccurate detectionMicrobiological testing/measurementDNA/RNA fragmentationMedicineNucleotide
The invention provides a nucleic acid diagnosis kit for detecting schistosoma japonicum. The kit provides a group of primers which have high sensitivity and high specificity and are used for detectingschistosoma japonicum, wherein the primers have nucleic acid sequences as shown in SEQ ID NO.1-2. The invention constructs real-time fluorescent quantitative PCR detection technology with high sensitivity and high specificity for schistosoma japonicum free nucleic acids, and the detection technology can be used for accurately and quickly detecting infection of schistosoma japonicum, determining whether a water body contains miracidia and cercariae or not and performing species identification on schistosoma japonicum.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Clone, expression and use of Schistosoma Japonicum signal transduction protein Sjwnt-4 gene
The invention discloses a Wnt family gene which is amplified for the first time from Schistosoma japonicum 19-day schistosomulum by utilization of RACE technology, wherein, sequence analysis indicates that: a complete coding frame of the gene comprises 1311bp, 436 amino acids coded; and theoretical molecular weight of 49.6kD. Homology analysis results indicate that: amino acid sequences of the gene have typical Wnt family protein characteristics; similarity of the amino acid sequences with amino acid sequences of Dugesia japonica and human Wnt4 is relatively 43 percent and 37 percent; the gene is presumed to be a Wnt4 gene of schistosome and named as Sjwnt4 (GenBanK Log-On No.: DQ643829). Real-time quantitative PCR analysis indicates that: the gene is expressed all in 14-day schistosomulum, 19-day schistosomulum, 31-day imago, 44-day female worms and 44-day male worms. The invention constructs a pronucleus expression vector pGEX-4T-2-Sjwnt4 of the gene and applies an Escherichia colisystem for expression; expression proteins exist in the form of inclusion bodies; Western blottings indicate that expression products can be identified by crude antigen immune serums of Schistosoma japonicum imago.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
RPA primer and method for detecting schistosoma japonicum katsurada
PendingCN112795659AAmplifyStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyRibosome
The invention discloses a RPA primer and a method for detecting schistosoma japonicum katsurada, particularly relates to a biological detection technology, and belongs to the technical field of molecular biology. A pair of specific primers is designed according to an Sj28S ribosome gene sequence of the schistosoma japonicum, and an RPA method for nucleic acid detection of the schistosoma japonicum is further established. The method can specifically amplify and detect the nucleic acid of the schistosoma japonicum katsurada, and the lowest detectable genome DNA concentration is 100 fg / mu L. The product has the advantages of high specificity, high sensitivity and high stability, can realize the index amplification of target fragments within 20 minutes, has low requirements on environmental equipment, is suitable for the detection of large-batch samples, and has a better field application prospect.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Schistosoma japonicum heat shock protein (SjHSP90) recombinant protein and application thereof to schistosomiasis diagnosis and treatment effect evaluation
The invention relates to a schistosoma japonicum heat shock protein (SjHSP90) recombinant protein and application thereof to schistosomiasis diagnosis and treatment effect evaluation and belongs to the field of immunology. The invention provides the preparation of the SjHSP90 recombinant protein, wherein the amino acid sequence of the SjHSP90 recombinant protein is shown as SEQIDNO.1. The SjHSP90 recombinant protein is further applied to schistosomiasis diagnosis and treatment effect evaluation. According to the invention, the SjHSP90 recombinant protein is applied to the schistosomiasis diagnosis for the first time; the SjHSP90 recombinant protein can be used for not only the schistosomiasis diagnosis and but also the treatment effect evaluation, so that the SjHSP90 recombinant protein has broad market space and good social and economic benefits.
Owner:JIANGSU INST OF PARASITIC DISEASES
Primer set, probe, kit and detection method for detecting schistosoma japonicum intermediate host oncomelania
ActiveCN111961737ANo pollution in the processLow costMicrobiological testing/measurementDNA/RNA fragmentationMicrobiologyIntermediate host
The invention provides a primer set, a probe, a kit and a detection method for detecting schistosoma japonicum intermediate host oncomelania, wherein the primer set comprises an upstream primer and adownstream primer; the upstream primer has a sequence shown as SEQ ID No. 1; and the downstream primer has a sequence shown in SEQ ID No. 2. The primer set, the probe, the kit and the detection methodhave characteristics of rapidness, simplicity, convenience, visibility, no pollution and low cost, can be used for on-site detection and instant detection, and can avoid occurrence of missed detection.
Owner:JIANGSU INST OF PARASITIC DISEASES
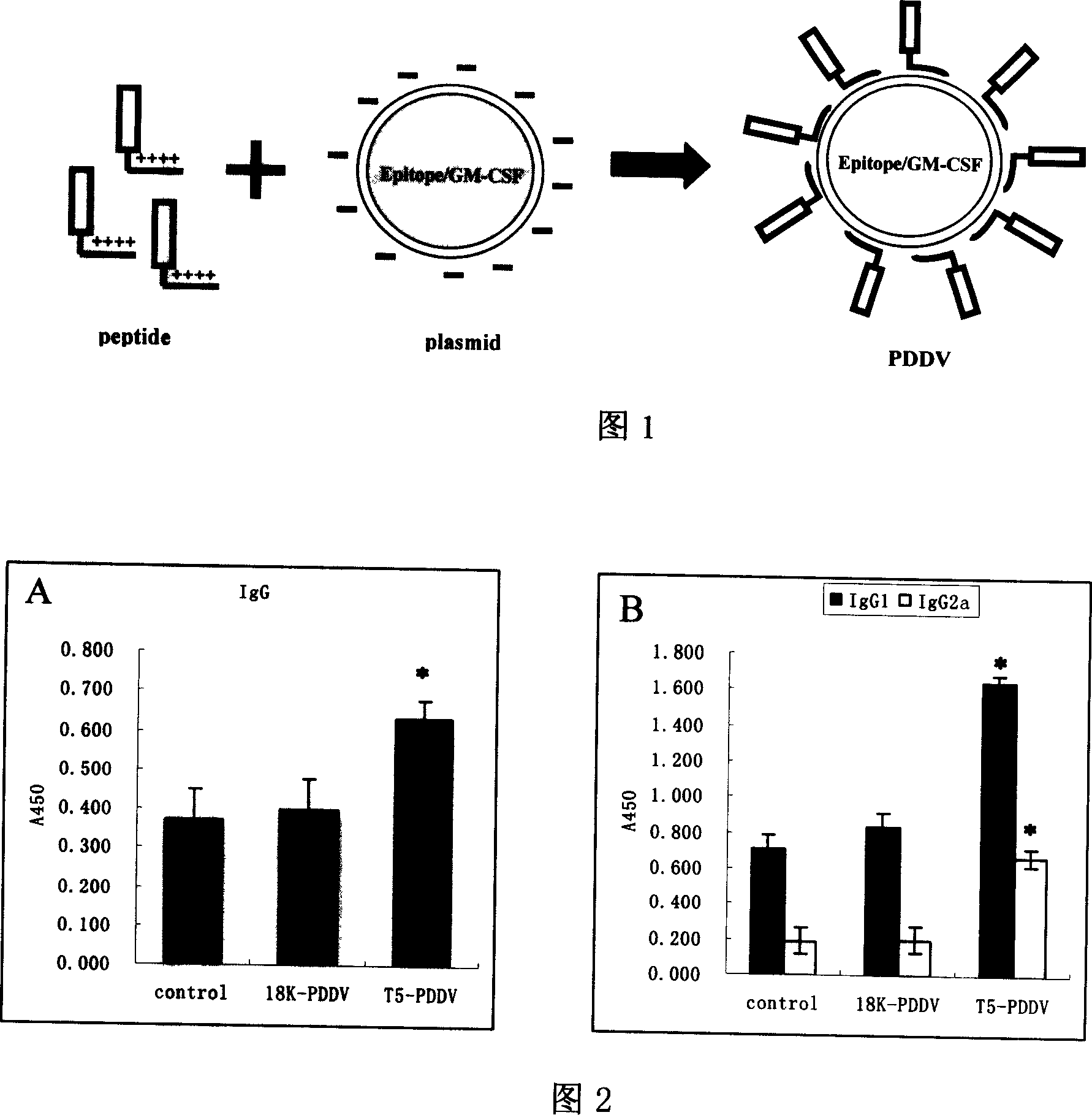
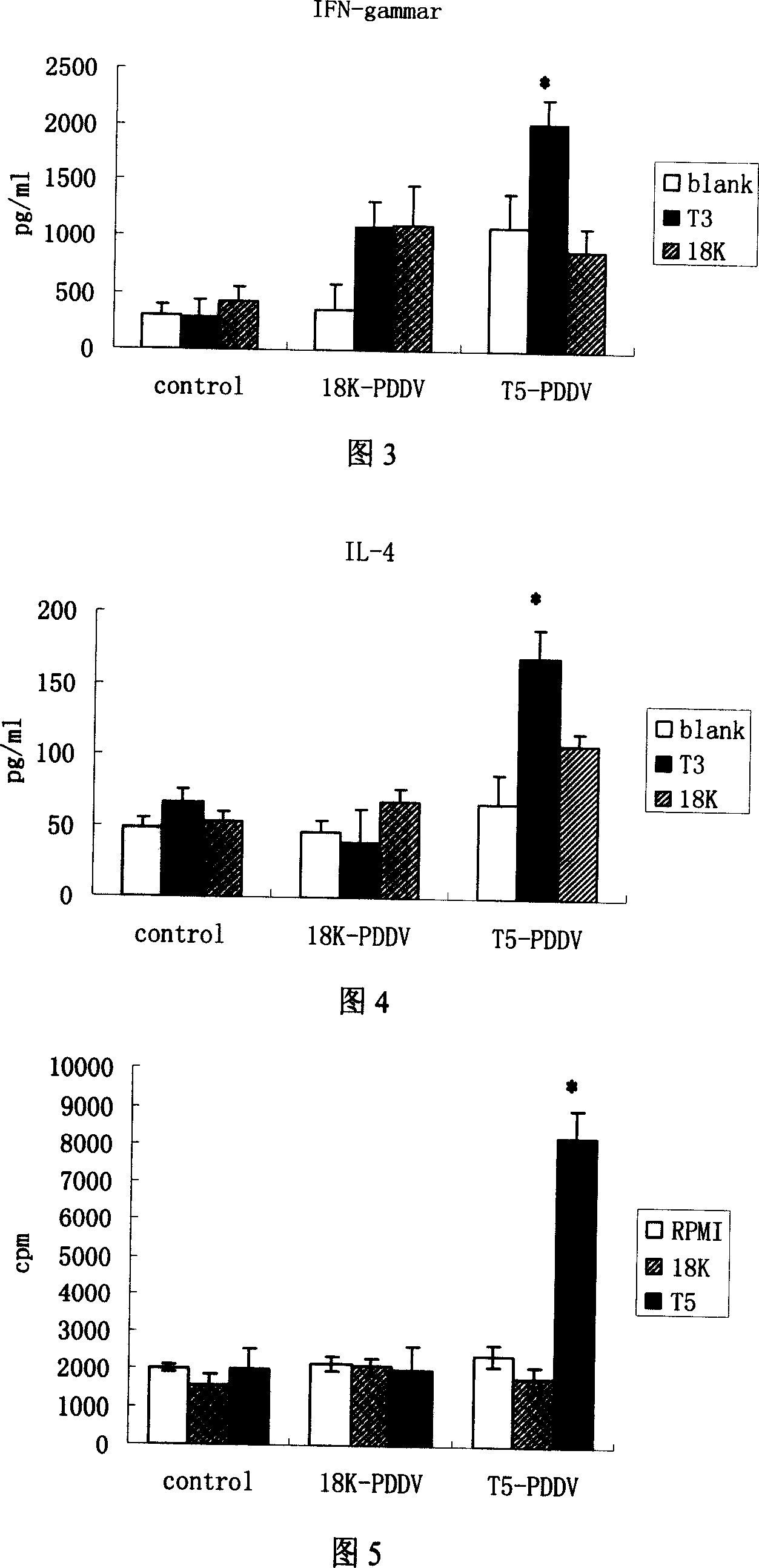
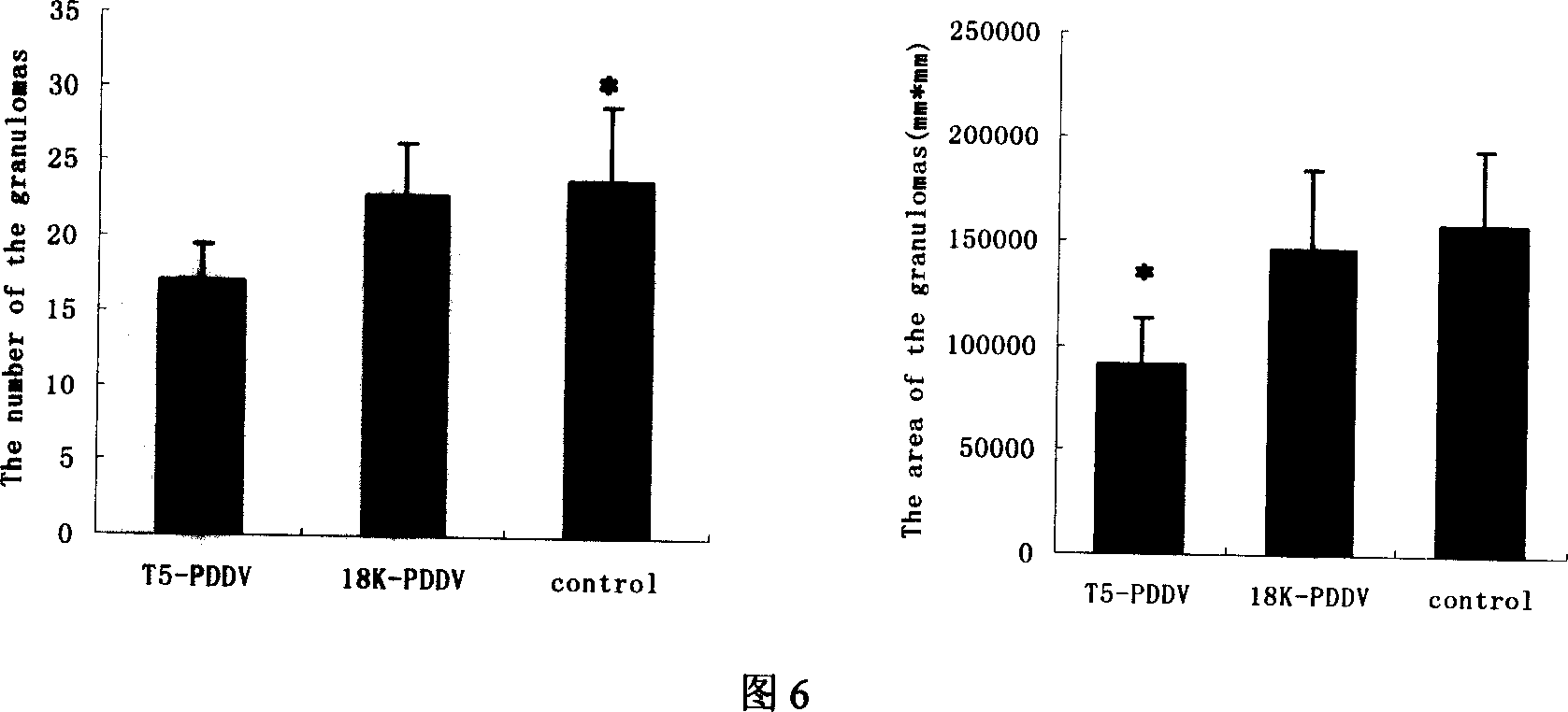
Peptide-DNA double vaccine based on T-cell epitope for anti-Schistosoma japonicum infection
InactiveCN101002948AImprove protectionStrong immunostimulatory abilityGenetic material ingredientsAntiparasitic agentsSchistosoma Japonicum InfectionFhit gene
A double peptide-DNA vaccine based on T-cell epitope for preventing and treating the Japanese schistosome infection features that its eucaryotic expression carrier carrying the epitope peptide coding gene is wrapped by the protein containing the epitope peptide. The amino acid sequence of said T-cell epitope peptide is AKQYNICCKFKELLD. Said vaccine can be prepared by cationic peptide carrying technique.
Owner:NANJING MEDICAL UNIV
Method for detecting infection with schistosoma japonicum by using host exosomes miRNA-223-3p
InactiveCN110760590AReduce traumaTraumaMicrobiological testing/measurementDNA/RNA fragmentationNucleotideMedicine
The invention discloses a method for detecting infection with schistosoma japonicum by using host exosomes. The nucleotide sequence of a serum exosome miR-223-3p is shown as SEQID NO:1, and the methodfor detecting infection with schistosoma japonicum is established according to a primer of which the nucleotide sequence is shown as SEQID NO:2. In accordance with the detection method of the schistosoma japonicum, the method is quick, acute, excellent and stable in detection. According to the method, the serum of patients is directly extracted, the wound parts of the patients can be alleviated,and the wound parts are small. A q-PCR detection method is used, so that the sensitivity is high, and the q-PCR detection method is high in sensitivity. An exosome extraction kit being mature exists,and besides, q-PCR detection is a common technique for laboratories, so that the method is simple relatively. Therefore, the method is worth of promotion, and has a good clinical application value.
Owner:SUN YAT SEN UNIV
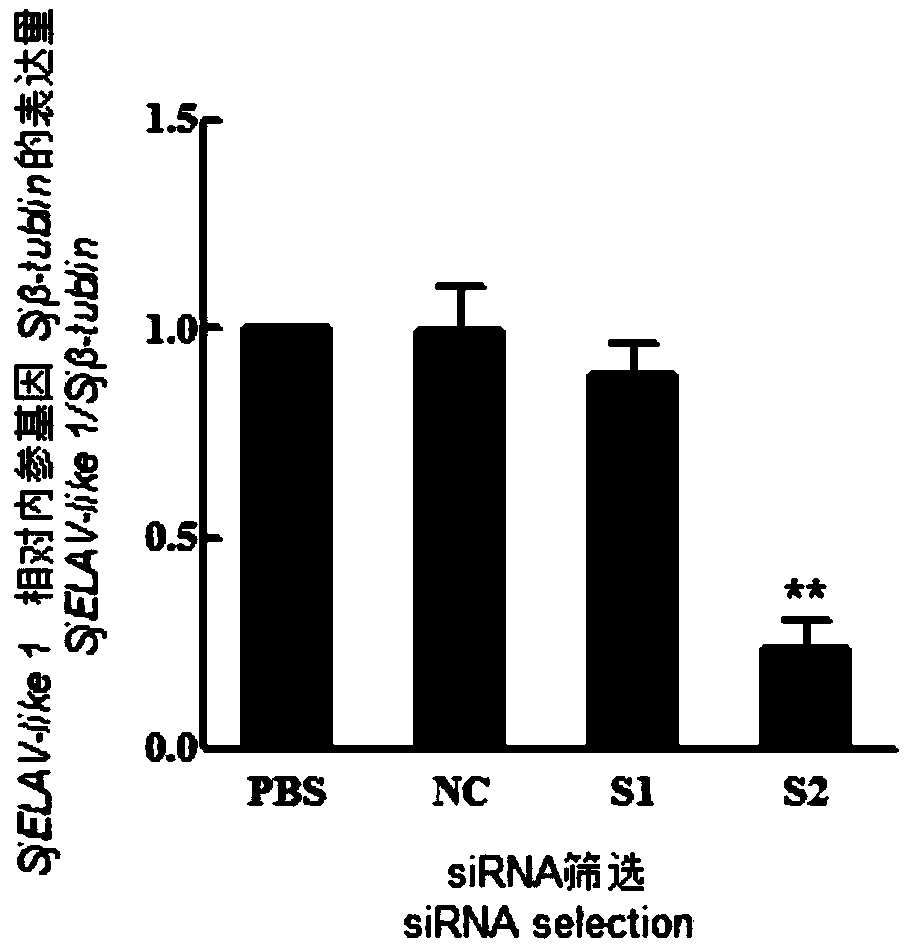
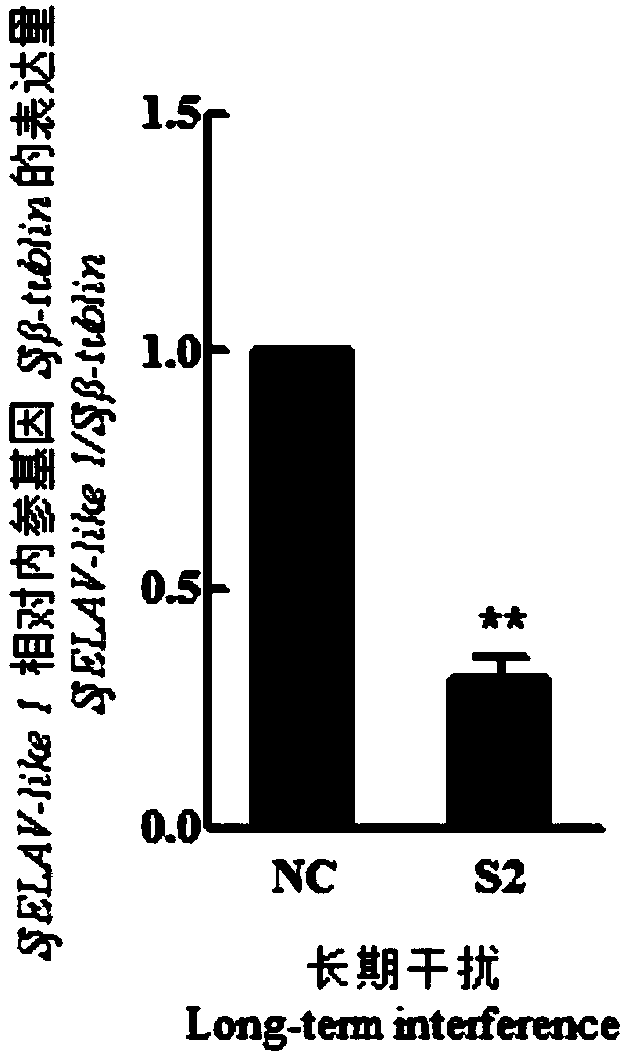
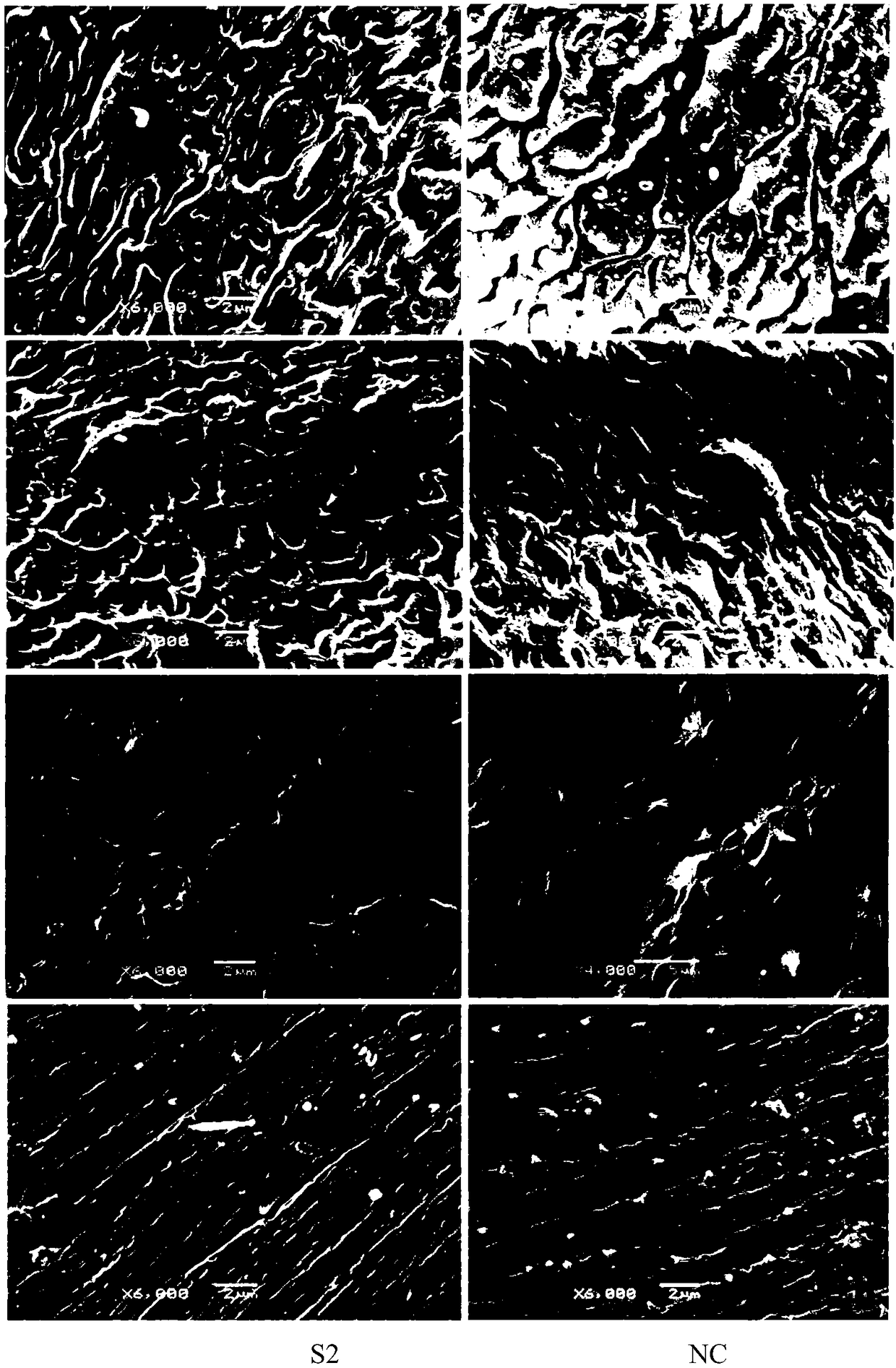
siRNA of schistosoma japonicum SjELAV-like 1 genes and application thereof
ActiveCN108795935ASuitable for preparationOrganic active ingredientsAntiparasitic agentsNucleotidePlant Germ Cells
The invention discloses siRNA of schistosoma japonicum SjELAV-like 1 genes. The siRNA has nucleotide sequences shown as SEQ ID NO.3 and SEQ ID NO.4. The invention further discloses the application ofthe siRNA of schistosoma japonicum SjELAV-like 1 genes. The siRNA of schistosoma japonicum SjELAV-like 1 genes disclosed by the invention is capable of obviously inhibiting transcription of the schistosoma japonicum SjELAV-like 1 genes and obviously reducing the number of liver loaded eggs and egg hatching rate of schistosomiasis infected mice, causes certain damages to the tegumental structure ofschistosoma and causes morphological abnormality of germ cells, and is applicable to preparation of drugs for treating schistosomiasis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Schistosoma japonicum recombinant antigen rsjmrp1 and its application
ActiveCN107778363BIncreased sensitivityStrong specificityBiological material analysisBiological testingAntigenSchistosomiases
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Fusion gene of Japan schistosome antigen gene and constituted DNA vaccin and preparing process
InactiveCN1904053BEfficient induction of immune protectionGood effectProtozoa antigen ingredientsMicrobiological testing/measurementAntigenSchistosomiases
The present invention discloses a fusion gene of schistosoma japonicum antigen genes, formed DNA vaccine and its preparation method. Said schistosoma japonicum DNA polyvalent vaccine is composed of schistosoma japonicum antigen genes (including sj23, sj FABP, sj26, sj GAPDSH, sj TPI and sj 97) and fusion gene of schistosoma japonicum antigen genes and eukaryotic expression vector with several insertion sites. The fusion gene of 30 schistosoma japonicum antigen genes is obtained by using 6 schistosoma japonicum antigen genes through 'fusion PCR' preparation process. Said fusion gene or antigengene can be inserted into eukaryotic expression vector so as to obtain the invented schistosoma japonicum DNA polyvalent vaccine.
Owner:HUAZHONG UNIV OF SCI & TECH
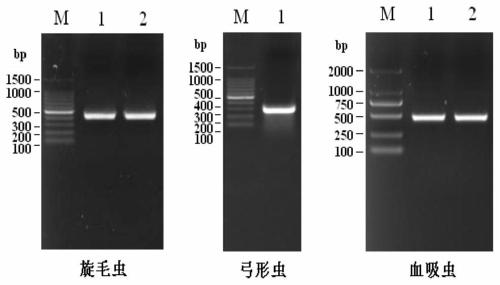
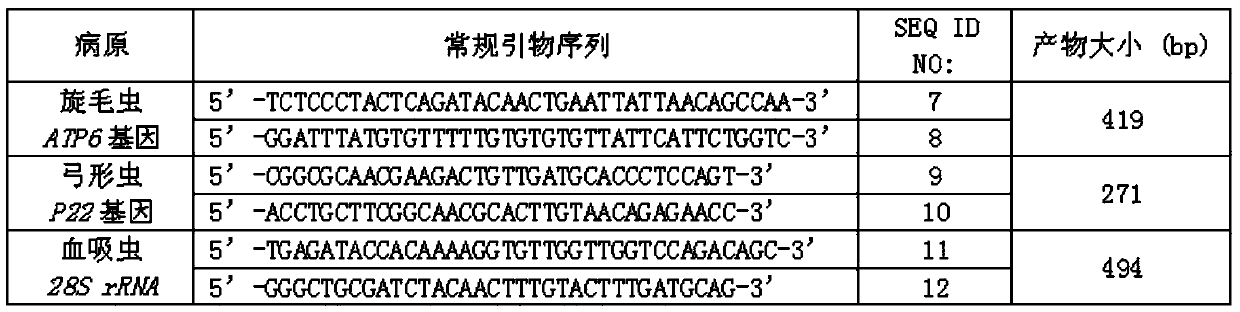
A kind of multiple dpo-pcr primer combination and method for detecting Trichinella spiralis, Toxoplasma gondii and Schistosoma
InactiveCN106701985BRealize qualitative detectionStrong specificityMicrobiological testing/measurementMicroorganism based processesMuscle tissueGondii toxoplasma
The invention provides a multiple DPO-PCR (dual priming oligonucleotide-polymerase chain reaction) primer combination for detecting trichinella spiralis, toxoplasma gondii and schistosome. The sequences of the primer combination are as shown in SEQ ID NO:1 to SEQ ID NO:6 correspondingly. The invention also provides a multiple DPO-PCR method for detecting the trichinella spiralis, the toxoplasma gondii and the schistosome. By adopting the method, qualitative detection on the muscular tissue or viscera sample of animals suffering from diseases can be realized. Experiments indicate that the detection method provided by the invention has high specificity, high flux and high speed, the detection efficiency is improved, and a more effective method is provided for detecting three animal origin parasitic zoonoses of the trichinella spiralis, the toxoplasma gondii and the schistosome.
Owner:海南出入境检验检疫局检验检疫技术中心
Schistosoma japonicum antigen protein rSjScP92 and application thereof
ActiveCN114805524AHigh detection sensitivityStrong specificityBiological material analysisAntiparasitic agentsDiseaseSchistosomiases
The invention provides a schistosoma japonicum antigen protein rSjScP92 and an application thereof. A series of genes which are highly expressed in schistosoma japonicum schistosoma are screened by using a schistosoma japonicum schistosoma whole genome expression profile chip, and antigen proteins coded by the genes SjScP15, SjScP57 and SjScP92 can be specifically recognized by serum of a schistosomiasis patient and show a relatively strong positive reaction. ELISA (enzyme-linked immunosorbent assay) detection shows that the antigen proteins have high sensitivity and specificity when being used for detecting the schistosoma japonicum and can be used for developing a schistosoma japonicum disease diagnostic reagent. After mice immunized by the schistosoma japonicum antigen proteins SjScP15, SjScP57 and SjScP92 are attacked by insects, the immune protection effect shows that the SjScP15, the SjScP57 and the SjScP92 are expected to be developed into schistosomiasis vaccines.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Method for detecting schistosoma japonicum infection by using host exosome miRNA-142a-3p
InactiveCN110760589AReduce traumaTraumaMicrobiological testing/measurementDNA/RNA fragmentationNucleotideMedicine
The invention discloses a method for detecting schistosoma japonicum infection by using a host exosome miRNA-142a-3p. A nucleotide sequence of the serum exosome miR-142a-3p is as shown in SEQ ID NO 1,and the method for detecting the schistosoma japonicum infection is established based on primers with a nucleotide sequence as shown in SEQ ID NO 2. The method for detecting the schistosoma japonicuminfection by using the host exosome miRNA-142a-3p is rapid, sensitive, specific and stable to detect. According to the method, serum of patients is directly extracted so that the trauma of the patients is reduced, and the trauma is small; a q-PCR detection method is used, and the sensitivity is high; and the q-PCR detection method has high sensitivity. At present, a mature exosome extraction kitis provided, meanwhile, q-PCR detection is a commonly used technology in a laboratory and is relatively simple. Therefore, the method is worth popularizing and has a good clinical application value.
Owner:SUN YAT SEN UNIV
Gene highly expressed in schistosoma japonicum katsurada, and coding protein and application thereof
ActiveCN112080506AHigh detection sensitivityStrong specificityBiological material analysisAntiparasitic agentsSchistosomiasesMicrobiology
The invention provides a gene highly expressed in schistosoma japonicum katsurada, and a coding protein and application thereof. A schistosoma japonicum whole genome expression profile chip is utilized to screen a series of genes highly expressed in schistosoma japonicum katsurada, wherein the antigen proteins coded by the genes SjScP27, SjScP80, SjScP84 and SjScP88 can be specifically recognizedby the serum of a schistosomiasis patient, and have strong positive reaction. The ELISA detection indicates that the antigen proteins have high sensitivity and specificity when being used for detecting schistosoma japonicum, and can be used for developing schistosoma japonicum diagnostic reagents.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com