Schistosoma japonicum antigen protein rSjScP92 and application thereof
A technology of antigenic protein and schistosomiasis, which is applied in the direction of anti-infective drugs, antibody medical ingredients, medical preparations containing active ingredients, etc., can solve the problem of clearing child worms, etc., and achieve the effect of simple operation, high repeatability, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
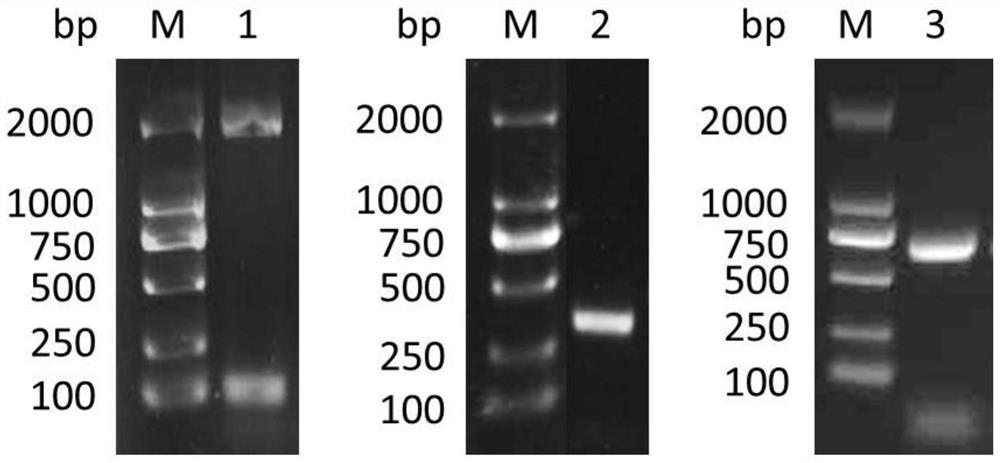
[0076] Example 1 Cloning of Schistosoma japonicum SjScP15, SjScP57 and SjScP92 genes
[0077] According to the SjScP15, SjScP57 and SjScP92 gene sequences found by the inventors by predicting the Schistosoma japonicum genome, primers were designed and restriction sites were introduced. Since the 190-400 nucleotides at the 3′ end of the SjScP57-encoded gene are highly conserved, the amino acid sequence of the encoded protein is highly homologous to human and other mammalian tyrosine hydroxylases, and the immune epitope-induced antibody of the encoded protein is very easy Cause non-specific reaction, on the one hand, it may cause non-specific reaction of antibodies, thus affecting the specificity of diagnosis; on the other hand, if the host is immunized with this protein segment, the induced antibody may non-specifically affect the activity of host tyrosine protease, which is not conducive to vaccines 's research and development. Therefore, in the present invention, a non-conse...
Embodiment 2
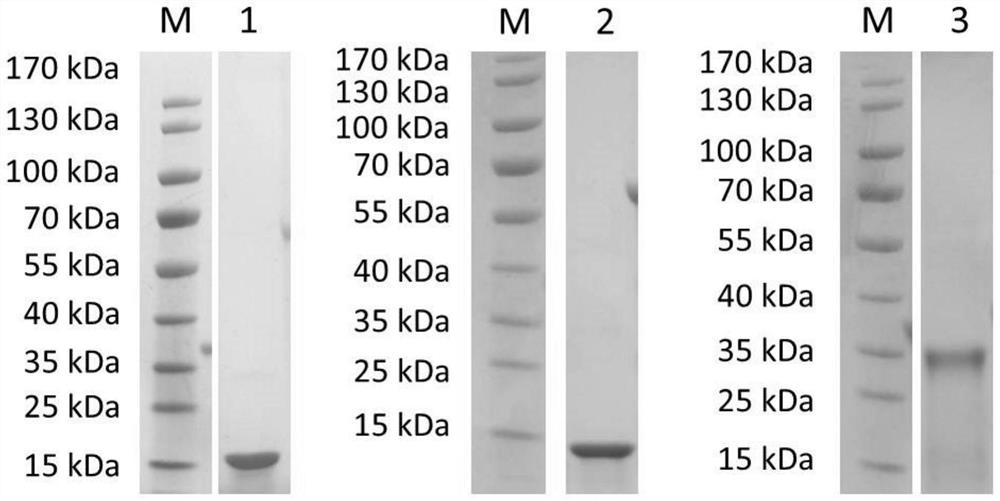
[0092] Example 2 Expression and purification of recombinant proteins of Schistosoma japonicum rSjScP15, rSjScP57 and rSjScP92
[0093] The clones identified as positive by the above PCR were inoculated into 15 mL of LB liquid medium (containing 50 μg / ml ampicillin), cultured at 37°C at 200 rpm overnight, and 10 mL of the medium was transferred into LB medium (containing 50 μg / ml ampicillin) the next day. Penicillin) 1L, continue to cultivate to OD 600nm The value was 0.8, then IPTG with a final concentration of 1 mM was added for induction, expression was performed at 18°C at 140 rpm for 16 hours, the cells were collected by centrifugation, and frozen at -80°C for use.
[0094] A small amount of bacteria before and after induction were resuspended in PBS buffer, added with SDS-PAGE loading buffer, mixed and boiled in a boiling water bath for 5 min to denature the protein.
[0095] 10 µl of the pre-induction and post-induction samples were added to each loading well for SDS-...
Embodiment 3
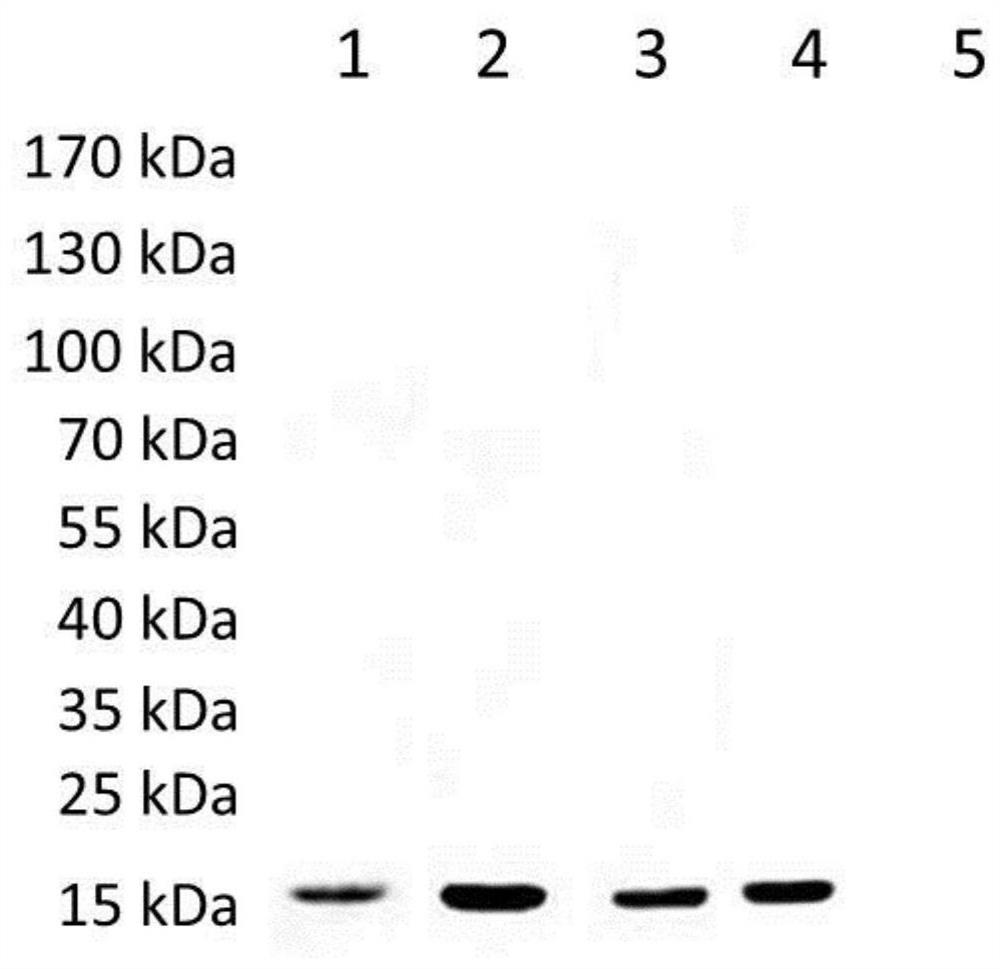
[0104] Example 3 Antigenicity detection of recombinant proteins of Schistosoma japonicum rSjScP15, rSjScP57 and rSjScP92
[0105] SDS-PAGE electrophoresis: take 100ng of recombinant protein for loading, and the electrophoresis conditions are: 100V for 20min, 120V for 1h.
[0106] Transfer membrane: The protein in the PAGE gel was transferred to the PVDF membrane by wet transfer method, and the electroporation conditions were: ice bath, 100V for 1h.
[0107] Blocking: The PVDF membrane was blocked with 5% nonfat milk powder at room temperature for 2 h, and washed three times with TBST buffer.
[0108] Incubation with primary antibody: serum of BALB / c mice infected with S. japonicum for 42 days, serum of New Zealand white rabbits infected with S. japonicum for 42 days, and serum of patients with S. japonicum were added respectively. Company) as a positive control and healthy mouse serum as a negative control (1:500 dilution with blocking solution), incubated overnight at 4°C, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com