Fusion gene of Japan schistosome antigen gene and constituted DNA vaccin and preparing process
A technology of DNA vaccine and antigen gene, which is applied in the field of polyvalent DNA vaccine of Schistosoma japonicum, can solve the problem of not being the most effective antigen form, and achieve the effects of scalable preparation, high separation efficiency, and convenience for storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0064][Example 1] Cloning of Schistosoma japonicum antigen genes sjFABP, sj23, sjGAPDH, sj26, sjTPI, sj97
[0065] 1. Extraction of total RNA from adult worms
[0066] 1) Collect adults from the portal vein of rabbits infected for 42 days with normal saline aortic perfusion;
[0067] 2) Take 100mg of fresh adult worms, add 1ml Trizol, quickly grind on ice with a glass homogenizer to make a homogenate, and place it at room temperature for 5min;
[0068] 3) Add 0.2ml chloroform, shake for 15S, and place at room temperature for 3min;
[0069] 4) Centrifuge at 12,000 rpm for 15 minutes at 4°C, and transfer the supernatant to another EP tube;
[0070] 5) Add 0.5ml of isopropanol, vortex and mix well, and let stand at room temperature for 10 minutes;
[0071] 6) Centrifuge at 12000rpm for 10min at 4°C to precipitate RNA and discard the supernatant;
[0072] 7) Add 1ml of 75% ethanol, centrifuge at 7500rpm for 5min at 4°C, and discard the supernatant;
[0073] 8) Dry the RNA in ...
example 2
[0091] (Example 2) Preparation of fusion antigen gene
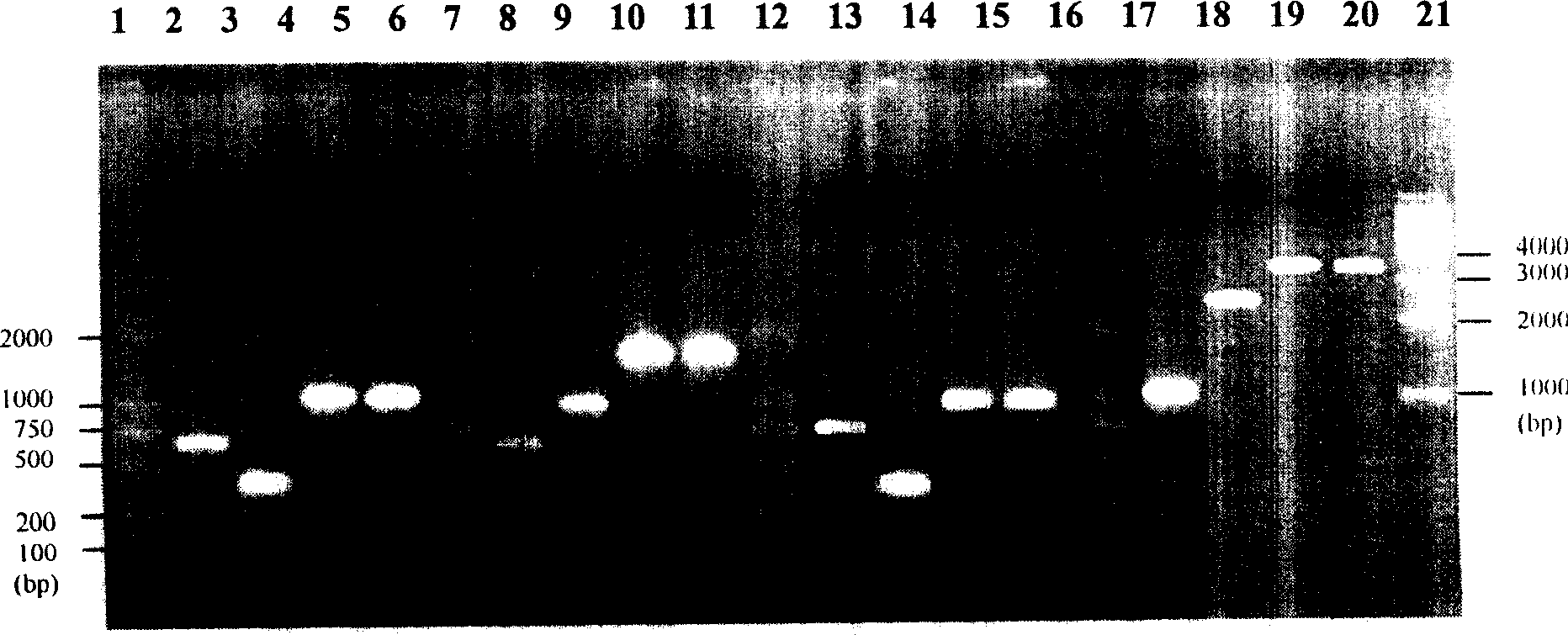
[0092] 1. Construction of fusion PCR hinge region primers and upstream and downstream primers required for fusion genes of 30 Schistosoma japonicum antigen genes, as shown in Table 2.
[0093] 2. Fusion PCR to prepare fusion genes of 30 Schistosoma japonicum antigen genes (taking sj23-FABP as an example, and so on for other 29 kinds)
[0094] Japanese blood
fluke resistance
Protogene
Reactant
volume
(μl)
PCR
sj23
10×PCR Buffer
MgCl 2 (25mM)
10mM dNTPs
pGEM-sj23
P1 (20μM)
P13 (20μM)
Taq DNA
Polymerase (2U / μl)
dd H 2 o
5.0
4.0
1.0
0.1
1.0
1.0
0.5
37.4
95℃5min→(94℃1min→45℃1min→72℃
1min) 35 cycles → 72°C 10min → 4°C storage
Japanese blood
...
example 3
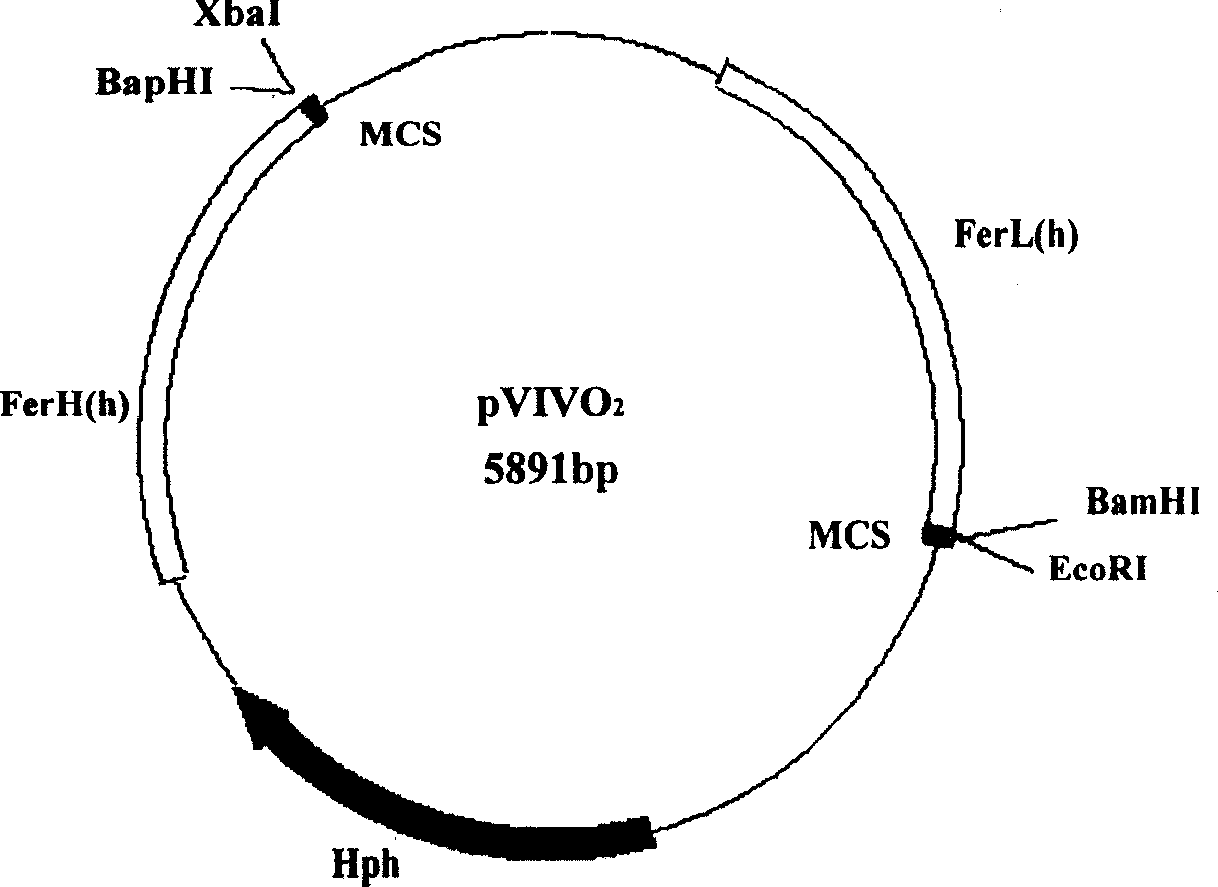
[0107] [Example 3] Schistosoma japonicum DNA multivalent vaccine pVIVO 2 / sjFABP-23, pVIVO 2 Construction of / sjFABP-23 / sjGAPDH-26
[0108] Fusion antigen gene fragment sjFABP-23, its 5' end contains BamHI restriction site Its 3' end contains an EcoRI restriction site The above T vector containing the sj FABP-23 fusion antigen gene was double digested with Bam HI and EcoR I, and the sjFABP-23 gene was recovered. Digest pVIVO with Bam HI and EcoR I 2 , recovery of pVIVO 2 fragment. Insertion of sjFABP-23 gene into pVIVO 2 Between BamHI and EcoRI, build strategy see attached Figure 4 . The specific operation is as follows:
[0109] 1. Build pVIVO 2 Recombinant plasmid of sjFABP-23:
[0110] The sjFABP-23 fragment DNA was double digested with restriction endonucleases EcoRI and BamHI. The reaction system is: 10×(Y + Tango TM )buffer 5μl, plasmid 5μg, BamHI 10U, EcoRI 10U, and water up to 50μl. After mixing, react in a 37°C water bath for 8-9 hours or overnight. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com