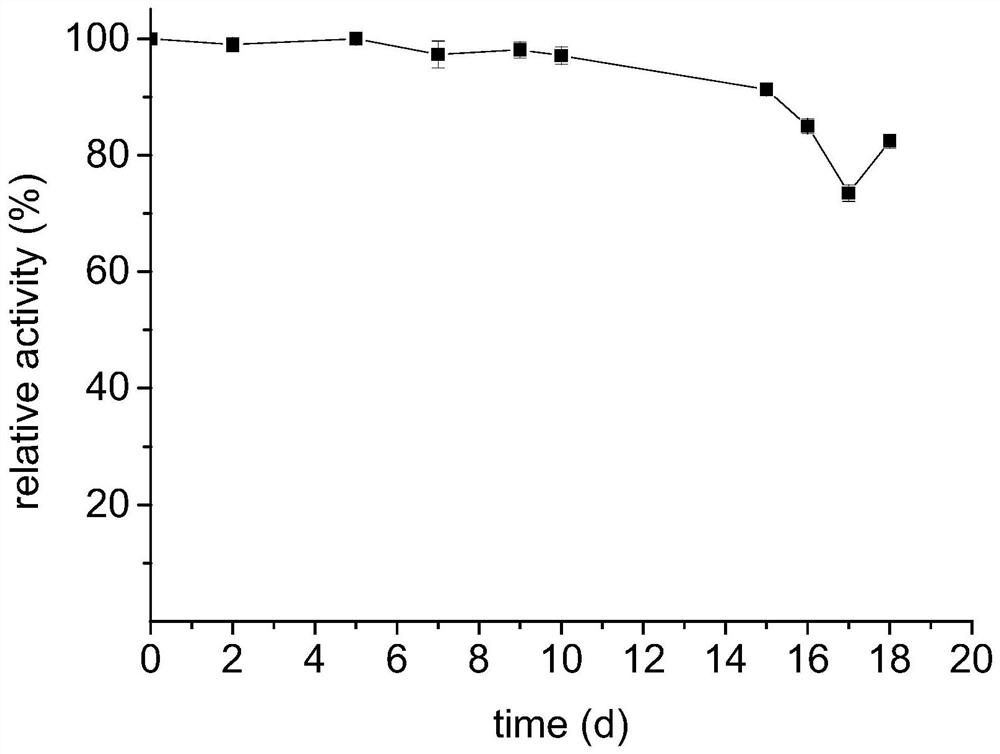
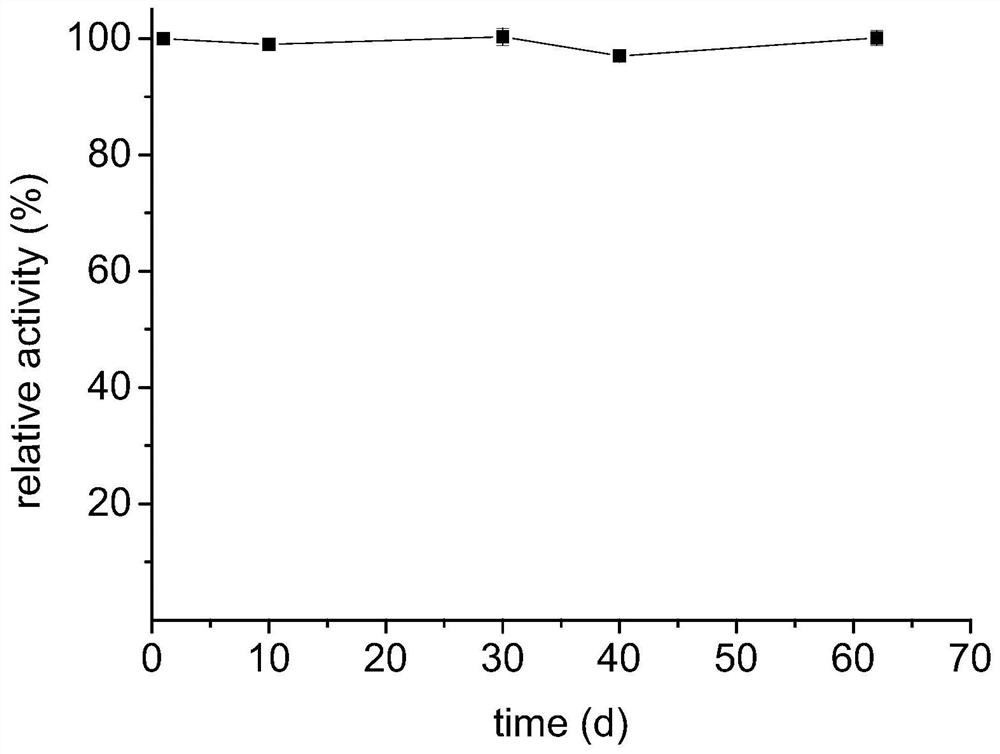
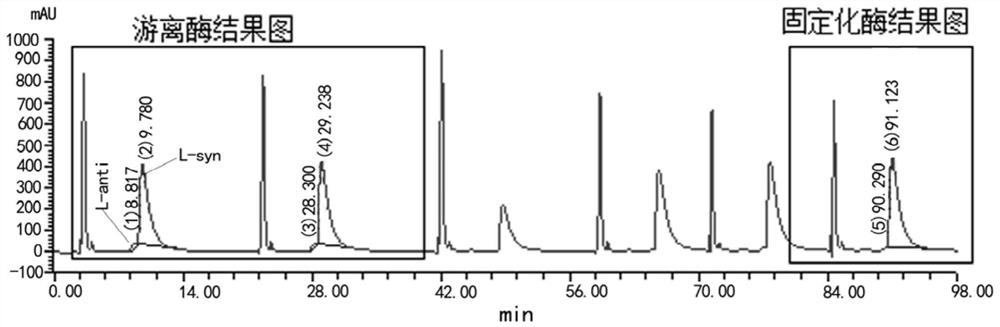
Preparation method of immobilized threonine aldolase, immobilized threonine aldolase and application
A technology of threonine aldolase enzyme and threonine aldolase enzyme liquid is applied in the field of immobilized threonine aldolase and its application, and the preparation field of immobilized threonine aldolase can solve the problem of Using resin and other problems to achieve huge economic and social benefits and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of free threonine aldolase enzyme solution
[0030] Use Escherichia coli BL21 engineering strains containing threonine aldolase gene (gene derived from Bacillus nealsonii, GenBank accession code WP_016204489.1) (for the construction method, please refer to the articles published by our laboratory W. Zheng, K. Chen, Z .Wang, X.Cheng, G.Xu, L.Yang, J.Wu, Organic Letters, 22(2020) 5763-5767.), Streak the plate in a clean bench and culture it in a 37°C incubator for 12h, Then pick the bacteria in the ultra-clean platform at 37 ° C, 200rpm shaker culture 2 ~ 3h, OD 600 At about 0.8 at 18°C, 200rpm induction culture for 16h to OD 600 At about 3, centrifuge at 4°C, 12000rpm for 10min, and discard the supernatant. Every 10 mL of bacterial liquid was centrifuged with 3 mL of 50 nM, pH 8.0 phosphate buffer to suspend, and then crushed at 400 W for 20 times to obtain free threonine aldolase enzyme liquid.
Embodiment 2
[0031] Embodiment 2: the pretreatment of resin
[0032] Wash a certain amount of amino resins NAA, HAA, HA, NH, ESQ-3 with 50mM, pH 8.0 phosphate buffer (when cleaning, use a glass rod to gently stir to prevent the resin from breaking) 2 or 3 times and no foam will appear . Mix the cleaned amino resin NAA and the glutaraldehyde crosslinking agent with a volume fraction of 0.4% at a ratio of mass (resin mass before cleaning): volume = 1:3, and activate it on a shaker at 25°C and 150rpm for 2-3h . The activated resin turns yellow from the original one, and the activated amino resin NAA is suction-filtered, and then washed with 50mM, pH 8.0 phosphate buffer for 2-3 times to remove the residual glutaraldehyde on the surface, then suction-filtered, and then used The freeze-dried and activated NAA was stored at 4°C for future use. Take a certain amount of D301, D301R, D354, and D314 and soak them in distilled water for 24 hours, then soak them in ethanol with a volume fraction of...
Embodiment 3
[0033] Example 3: Immobilization of threonine aldolase
[0034] Add the activated resin and free threonine aldolase enzyme solution to 10mL of 50mM, pH 8.0 phosphate buffer solution at a ratio of 0.6g carrier / 3mL free enzyme solution and place in a shaker at 30°C and 150rmp Immobilized for 4 to 5 hours. Then the obtained immobilized enzyme was washed 2 to 3 times to remove the residual enzyme on the surface, filtered by suction, freeze-dried and stored at 4°C for future use.
[0035]Sodium alginate immobilized enzyme: dissolve (pH 8.0 phosphate buffer solution) to prepare 7mL of sodium alginate with a concentration of 3%, place it in a bath at 40-50°C and heat to ensure that the sodium alginate is fully dissolved, add 3mL after cooling For the foam breaking solution, stir and dissolve evenly with a glass rod. Use a syringe to absorb the dissolved sodium alginate enzyme solution, and slowly add it dropwise to 3% 50mL calcium chloride solution to form sodium alginate embedding...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap