Preparation method of brain protective agent and key impurities thereof
A brain protection and impurity technology, which is applied in the preparation of hydrazone, muscular system diseases, neuromuscular system diseases, etc., and can solve the problem of inaccurate control of process conditions such as temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
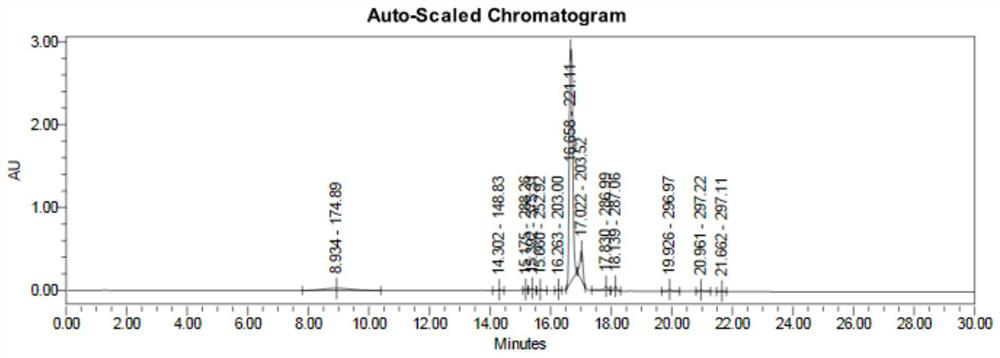
[0037] (1) Add 108 g of phenylhydrazine to 324 mL of ethanol, add 136.5 g of ethyl acetoacetate at a temperature of -5 to 0 ° C, and react at a temperature of -5 ° C for 1 hour. After the reaction, half of the reaction solution is taken out and added with a mass fraction of 20% The reaction was quenched with 100 mL of sodium bicarbonate solution, extracted with dichloromethane, concentrated under reduced pressure at 35°C to obtain an oil, purified by column chromatography, and dried to obtain 96.59 g of transition state impurities with a yield of 87.7%. LC-MS showed that the [M+H] at 16.658min was 221.11, consistent with the molecular weight of the target product, and determined to be a transition state impurity.
[0038] Raise the temperature of the remaining half of the reaction solution to 60-65°C for 1 hour, quickly add 5.4g of concentrated sulfuric acid solution, raise the temperature to 80-85°C, and react for 3 hours under temperature control. Lavone 78.91g, yield 90.6%....
Embodiment 2
[0042] (1) Add 108 g of phenylhydrazine to 540 mL of isopropanol, add 136.5 g of ethyl acetoacetate at a temperature of -5 to 0 ° C, and react at a temperature of -5 ° C for 1 hour. After the reaction, half of the reaction solution is taken out and added with a mass fraction of Quench the reaction with 100 mL of 20% sodium bicarbonate solution, add dichloromethane for extraction, concentrate under reduced pressure at 35°C to obtain an oil, purify by column chromatography, and dry to obtain 93.50 g of transition state impurities with a yield of 84.9%.
[0043] Raise the temperature of the remaining half of the reaction solution to 60-65°C for 1 hour, quickly add 10.8 g of concentrated sulfuric acid solution, raise the temperature to 80-85°C, and react for 3 hours under temperature control. Lavone 79.35g, yield 91.1%.
[0044] (2) get step (1) gained Edaravone 17.4g to be dissolved in 104mL acetic acid solvent, add 68.0g mass concentration and be 50% hydrogen peroxide, react at ...
Embodiment 3
[0047](1) Add 108 g of phenylhydrazine to 864 mL of ethanol, add 136.5 g of ethyl acetoacetate at temperature control -5 to 0 ° C, and react at temperature -5 ° C for 1 hour. After the reaction, half of the reaction solution is taken out and added with a mass fraction of 20% carbonic acid Quench the reaction with 100 mL of sodium hydrogen solution, add dichloromethane for extraction, and concentrate under reduced pressure at 35°C to obtain an oil, which is purified by column chromatography and dried to obtain 99.76 g of transition state impurities with a yield of 90.4%.
[0048] Raise the temperature of the remaining half of the reaction solution to 60-65°C for 1 hour, quickly add 16.2 g of concentrated sulfuric acid solution, raise the temperature to 80-85°C, and react for 3 hours under temperature control. Lavone 78.04g, yield 89.6%.
[0049] (2) get step (1) gained Edaravone 17.4g to be dissolved in 139mL acetic acid solvent, add 102.0g mass concentration and be 50% hydroge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com