Liquid preparation of immunosuppressant monoclonal antibody
A monoclonal antibody and immunosuppressant technology, applied in the direction of antibodies, immunoglobulins, antibody medical components, etc., can solve the problems of many excipients and complicated operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
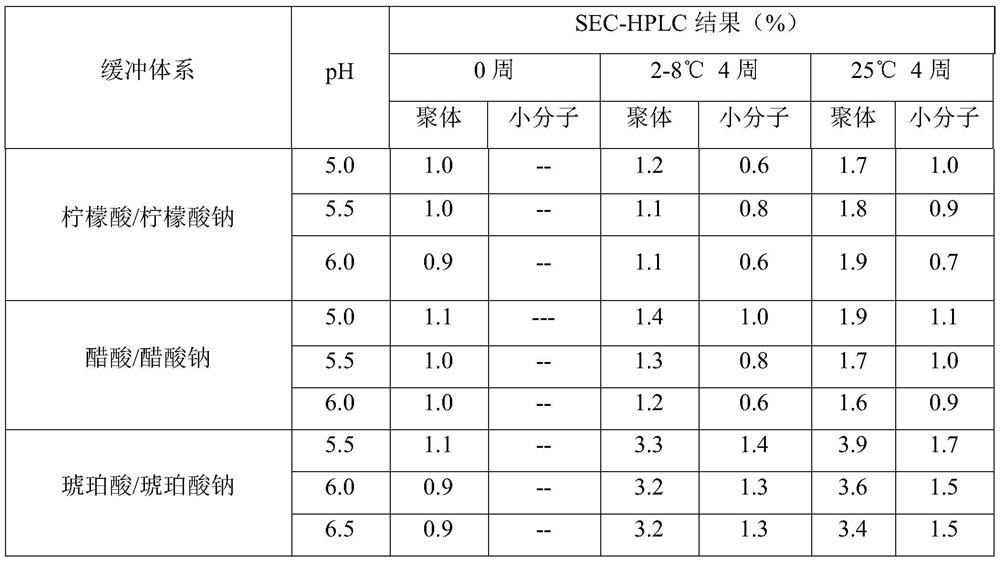
[0037] Embodiment 1 investigates the influence of buffer system on the stability of liquid formulations
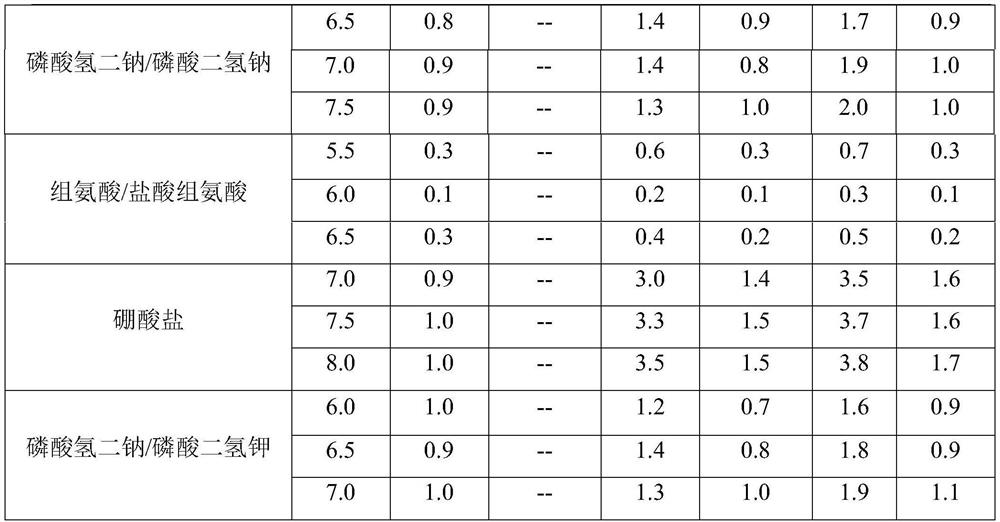
[0038] Combining the molecular characteristics of the anti-CD20 human-mouse chimeric monoclonal antibody, the buffer system commonly used in the protein preparation process, and the buffer range of the buffer system, after preliminary selection, citric acid / sodium citrate, acetic acid / sodium acetate, and disodium hydrogen phosphate were finally selected / sodium dihydrogen phosphate, histidine / histidine hydrochloride and disodium hydrogen phosphate / potassium dihydrogen phosphate buffer system were screened, and the preparation formula and test results are shown in Table 1 and Table 2, respectively.
[0039] Table 1 Liquid preparations of anti-CD20 human-mouse chimeric monoclonal antibody containing different buffer systems
[0040] serial number Element Formulation 1 Anti-CD20 Human Mouse Chimeric Monoclonal Antibody 20mg / mL 2 Polysorbate 80 0.003%...
Embodiment 2
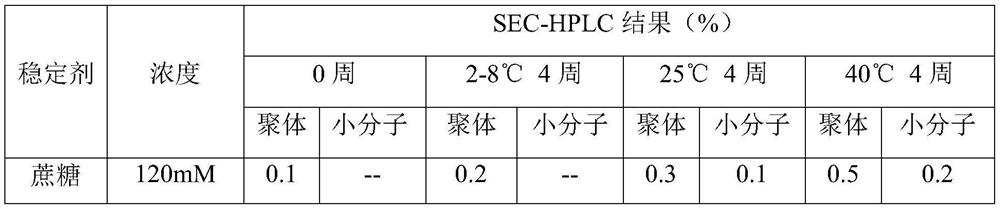
[0045] Embodiment 2 investigates the influence of stabilizer on the stability of liquid preparation
[0046] The preparation formula and test results are shown in Table 3 and Table 4, respectively.
[0047] Table 3 contains anti-CD20 human-mouse chimeric monoclonal antibody liquid preparations with different stabilizers
[0048] serial number Element Formulation 1 Anti-CD20 Human Mouse Chimeric Monoclonal Antibody 20mg / mL 2 Histidine / Histidine Hydrochloride 15mM 3 stabilizer See Table 4 4 Polysorbate 80 0.03% 5 Preparation pH 6.0
[0049] Table 4 SEC-HPLC aggregate results of anti-CD20 human-mouse chimeric monoclonal antibodies containing different stabilizers
[0050]
[0051]
[0052] Various liquid preparations added with different stabilizers were stored at 2-8°C, 25°C and 40°C respectively. The SEC-HPLC results showed that the increase of polymers and small molecules in the liquid preparations of 120mM and 240m...
Embodiment 3
[0053] Embodiment 3 investigates the influence of solubilizing agent on the stability of liquid preparation
[0054] The formulation formulations and stability test results are shown in Table 5 and Table 6, respectively. The liquid formulations of different formulations were subjected to 5 cycles of freezing and thawing and shaking for 3 days.
[0055] Table 5 Anti-CD20 human-mouse chimeric monoclonal antibody liquid preparations added with different concentrations of solubilizer
[0056] serial number Element Formulation 1 Anti-CD20 Human Mouse Chimeric Monoclonal Antibody 20mg / mL 2 Histidine / Histidine Hydrochloride 15mM 3 sucrose 120mM 4 Solubilizers See Table 6
[0057] Table 6 Freezing-thawing and shaking test results of anti-CD20 human-mouse chimeric monoclonal antibody liquid preparations added with different concentrations of solubilizer
[0058]
[0059]
[0060] Note: A-3D = shaking for 3 days, FT-5C = repeated f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com