Composite sodium hyaluronate gel capable of resisting hyaluronidase hydrolysis and preparation method of composite sodium hyaluronate gel
A technology of hyaluronidase and sodium hyaluronate, used in pharmaceutical formulations, medical science, prostheses, etc., can solve the problems of reduced biocompatibility, easy to cause immune response, etc., to delay degradation time, strengthen anti-enzyme Degradation ability, the effect of inhibiting enzymatic degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A chondroitin sulfate-sodium hyaluronate composite gel resistant to hyaluronidase hydrolysis, specifically prepared by the following method:
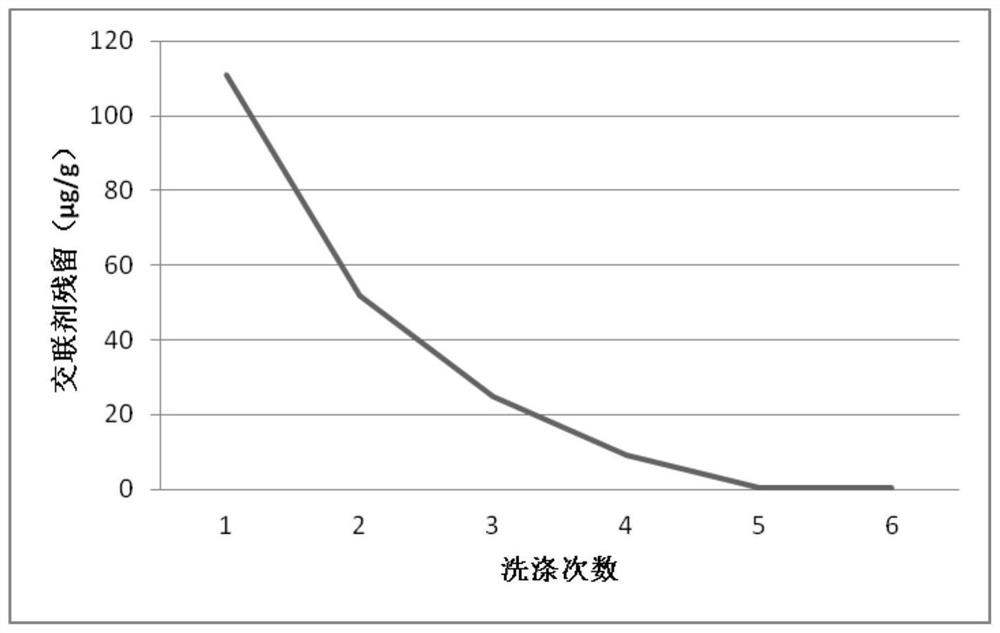
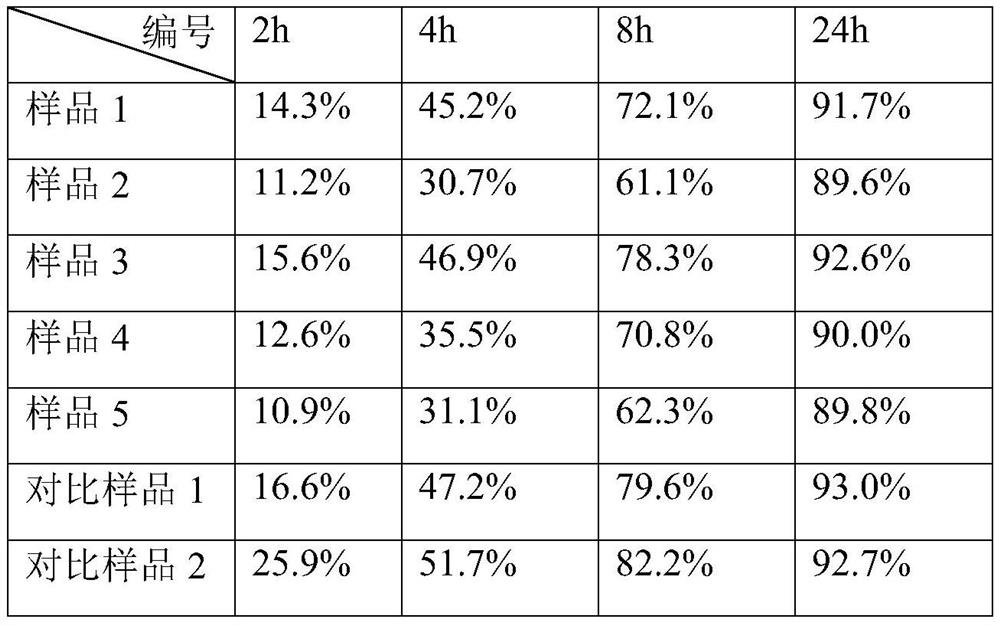
[0030] Weigh 1g of chondroitin sulfate (molecular weight: 10kDa), dissolve it in 50mL of NaOH solution (pH = 11) and keep stirring, add 5g of sodium hyaluronate dry powder (molecular weight: 800kDa), then add 1mL of BDDE and stir evenly. React for 4 hours in an environment with a temperature of 37° C., and obtain a primary cross-linked gel after the reaction is completed. The gel was precipitated with 95% ethanol, and the precipitate was repeatedly washed with purified water and then dried to obtain a gel powder.
[0031] Weigh 10g sodium hyaluronate dry powder (molecular weight 1500kDa) and dissolve it in 50mL NaOH solution (pH=11), then add 2g primary cross-linked gel dry powder and stir evenly, and stir and react at 37°C for 5h. After the reaction was completed, 1 mL of BDDE was added and stirred evenly to carry out secondary...
Embodiment 2
[0034] A chondroitin sulfate-sodium hyaluronate composite gel resistant to hyaluronidase hydrolysis, specifically prepared by the following method:
[0035] Weigh 2g of chondroitin sulfate (molecular weight: 10kDa), dissolve it in 50mL of NaOH solution (pH=11) and keep stirring, add 5g of sodium hyaluronate dry powder (molecular weight: 800kDa), add 1mL of BDDE and stir evenly. React for 4 hours in an environment with a temperature of 37° C., and obtain a primary cross-linked gel after the reaction is completed. The gel was precipitated with 95% ethanol, and the precipitate was repeatedly washed with purified water and then dried to obtain a gel powder.
[0036] Weigh 10g sodium hyaluronate dry powder (molecular weight 1500kDa) and dissolve it in 50mL NaOH solution (pH=11), then add 2g primary cross-linked gel dry powder and stir evenly, and stir and react at 37°C for 5h. After the reaction was completed, 1 mL of BDDE was added and stirred evenly to carry out secondary cross-li...
Embodiment 3
[0038] A chondroitin sulfate-sodium hyaluronate composite gel resistant to hyaluronidase hydrolysis, specifically prepared by the following method:
[0039] Weigh 2g of chondroitin sulfate (molecular weight: 10kDa), dissolve it in 50mL of NaOH solution (pH=11) and keep stirring, add 5g of sodium hyaluronate dry powder (molecular weight: 800kDa), add 1mL of BDDE and stir evenly. React for 4 hours in an environment with a temperature of 37° C., and obtain a primary cross-linked gel after the reaction is completed. The gel was precipitated with 95% ethanol, and the precipitate was repeatedly washed with purified water and then dried to obtain a gel powder.
[0040] Weigh 10g sodium hyaluronate dry powder (molecular weight 1500kDa) and dissolve it in 50mL NaOH solution (pH=11), then add 2g primary cross-linked gel dry powder and stir evenly, and stir and react at 37°C for 5h. After the reaction was completed, 1 mL of BDDE was added and stirred evenly to carry out secondary cross-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com