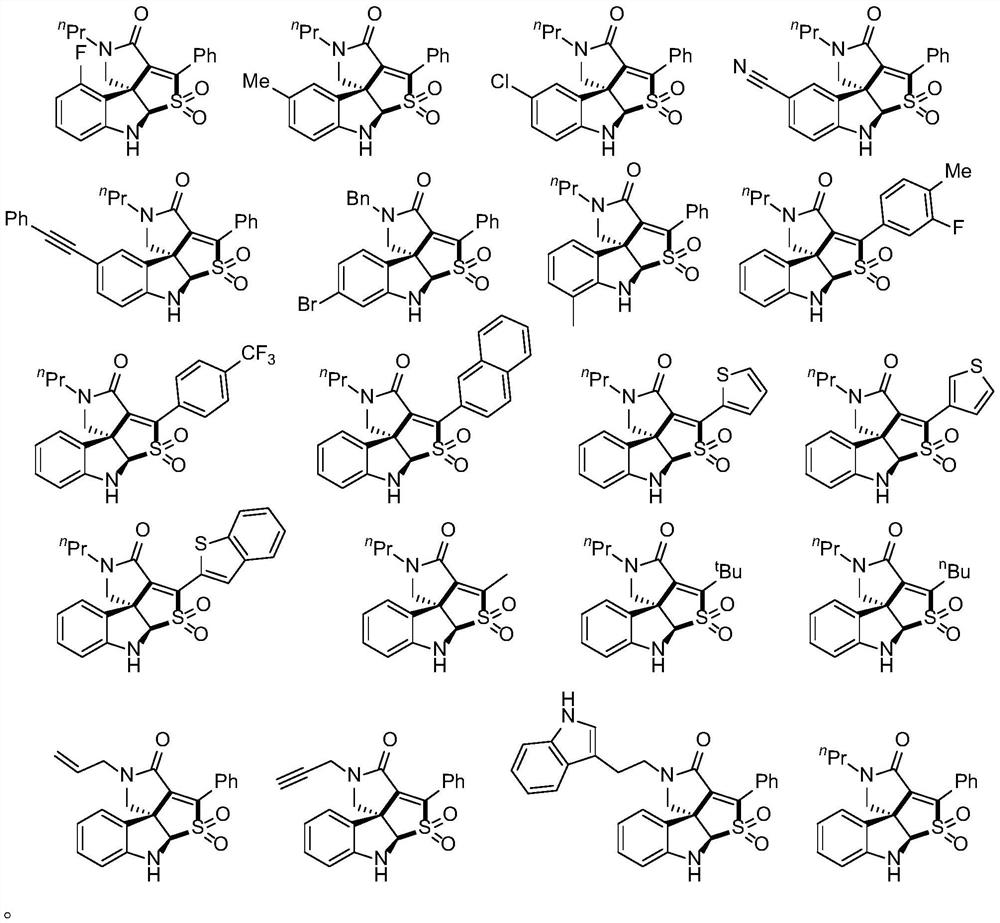
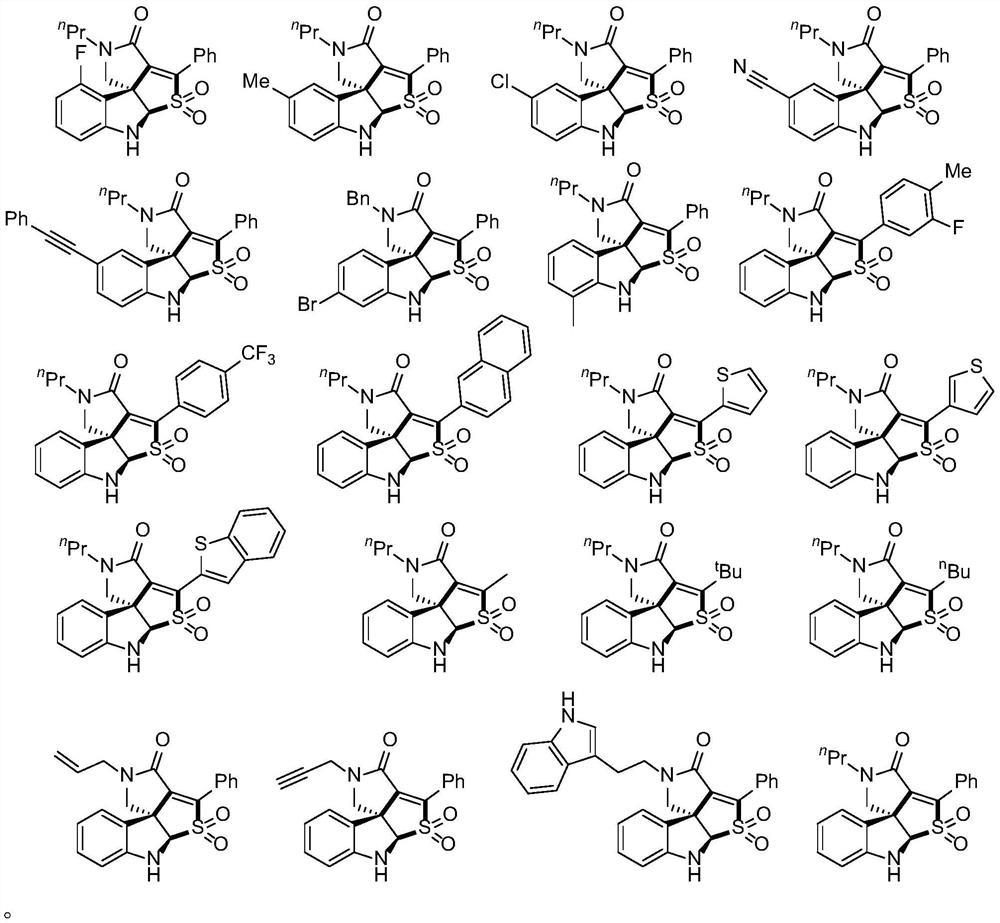
Method for preparing tetracyclic spiroindoline compound
A technology for indolines and compounds, which is applied in the field of preparing tetracyclic spirocyclic indolines, can solve the problems of limited reaction yields in the range of substrates, and achieve the effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A tetracyclic spirocyclic indoline compound, the preparation method is as follows:
[0036] Add 20-40mL of dichloroethane to a 100mL one-necked flask, and then add 10mmol of NaHSO 3 and 10mmol of 15mmol of lithium chloride and 0.2mmol of Rhodamine 6G were reacted under light at room temperature for 24 hours, and the solvent was removed under reduced pressure to obtain a crude product, which was then separated by flash column chromatography to obtain the product 72.6 mg (91%). The structure of the product characterizes the physical constants: 1 H NMR (500MHz, Chloroform-d) δ8.05–7.99(m,2H),7.48–7.39(m,3H),7.15(m,1H),6.62(d,J=8.0Hz,1H),6.55( d,J=9.0Hz,1H),5.52(s,1H),5.02(s,1H),3.91(d,J=10.0Hz,1H),3.74(d,J=10.0Hz,1H),3.47( m,1H),3.37(m,1H),1.66–1.59(m,2H),0.96(t,J=7.5Hz,3H). 13 C NMR (126MHz, Chloroform-d) δ158.9 (d, J=249.1Hz), 157.9, 149.7 (d, J=7.9Hz), 144.5, 136.8, 132.0 (d, J=8.8Hz), 131.2, 129.9 ,128.5,125.2,115.4(d,J=20.4Hz),108.1(d,J=20.7Hz),106.5(d,J=3.1Hz...
Embodiment 2
[0038] A tetracyclic spirocyclic indoline compound, the preparation method is as follows:
[0039] Add 20-40mL of dichloroethane to a 100mL one-necked flask, and then add 10mmol of NaHSO 3 and 10mmol of 15mmol of lithium chloride and 0.2mmol of Rhodamine 6G were reacted under light at room temperature for 24 hours, and the solvent was removed under reduced pressure to obtain a crude product, which was then separated by flash column chromatography to obtain the product 55.1 mg (70%). The structure of the product characterizes the physical constants: 1 H NMR (500MHz, Chloroform-d)δ8.00(m,2H),7.42(m,3H),7.02–6.92(m,2H),6.72(d,J=8.0Hz,1H),5.29(s, 1H), 5.00(s, 1H), 3.85(d, J=9.5Hz, 1H), 3.65(d, J=9.5Hz, 1H), 3.51(dt, J=14.5, 7.5Hz, 1H), 3.41( dt,J=14.0,7.5Hz,1H),2.23(s,3H),1.64(m,2H),0.98(t,J=7.5Hz,3H). 13 C NMR (126MHz, Chloroform-d) δ162.7, 144.6, 143.5, 137.5, 131.0, 130.8, 129.8, 129.7, 128.5, 125.3, 122.1, 110.6, 84.1, 58.2, 54.4, 45.6, 20.9, 20.7, 11.5.
Embodiment 3
[0041] A tetracyclic spirocyclic indoline compound, the preparation method is as follows:
[0042]Add 20-40mL of dichloroethane to a 100mL one-necked flask, and then add 10mmol of NaHSO 3 and 10mmol of 15mmol of lithium chloride and 0.2mmol of Rhodamine 6G were reacted under light at room temperature for 24 hours, and the solvent was removed under reduced pressure to obtain a crude product, which was then separated by flash column chromatography to obtain the product 66.4 mg (80%). The structure of the product characterizes the physical constants: 1 H NMR (500MHz, Chloroform-d) δ8.03–7.97(m,2H),7.50–7.41(m,3H),7.16(m,1H),7.12(d,J=2.0Hz,1H),6.74( d,J=8.5Hz,1H),5.43(s,1H),5.02(s,1H),3.86(d,J=9.5Hz,1H),3.66(d,J=9.5Hz,1H),3.58– 3.37(m,2H),1.64(m,3H),0.99(t,J=7.5Hz,3H). 13 C NMR (126MHz, Chloroform-d) δ162.3, 145.4, 144.2, 136.7, 131.2, 131.1, 130.3, 129.7, 128.6, 125.9, 125.1, 122.0, 111.6, 83.9, 58.0, 54.2, 45.6, 20.7, 11.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com