Method for preparing benzo-6, 8-dihydroisoquinoline-1-selenious sulfuryl benzamide compound through bimetallic catalysis
A technology of sulfone benzamide and dihydroisoquinoline, which is applied in the field of chemical intermediate preparation and can solve problems such as non-conformity, destruction, and low reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of product 4a
[0037] At room temperature, add 10mmol of 1,2,3,4-tetrahydroisoquinoline, 15mmol of sodium selenide and 10mmol of 2-bromobenzaldehyde in a 50mL round bottom flask, then add 30mL of DMSO and 1mmol of acetic acid Magnesium, 1 mmol of copper acetate, and 20 mmol of cesium carbonate were stirred at 100° C. for 24 hours. After cooling, add 20 mL of saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 20 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain The pure product of benzo-6,8-dihydroisoquinoline-1-selensulfonylbenzamide compound 4a.
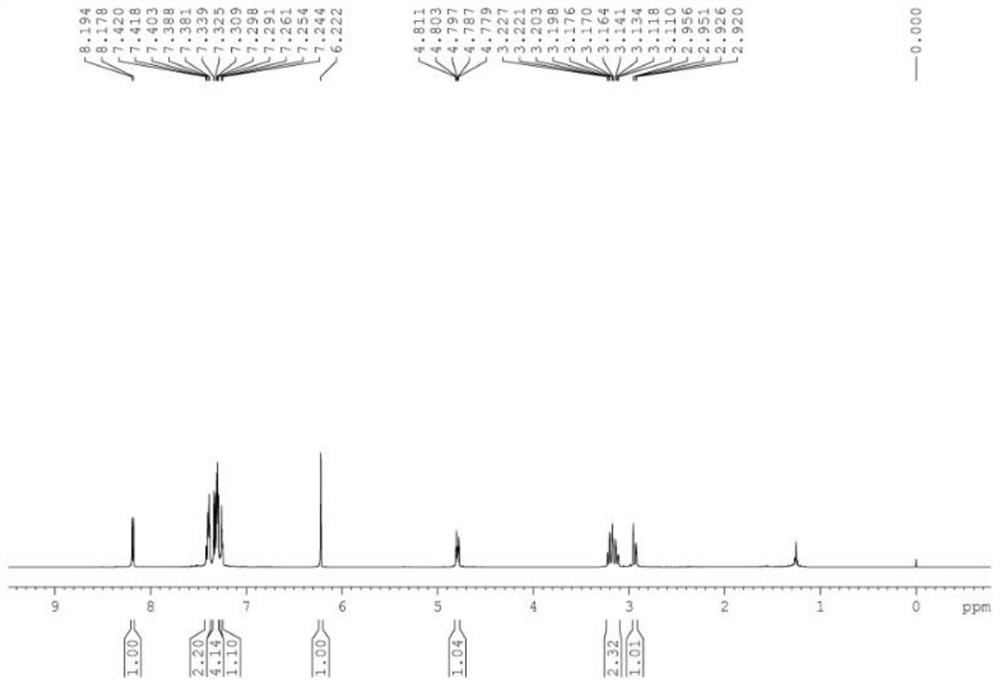
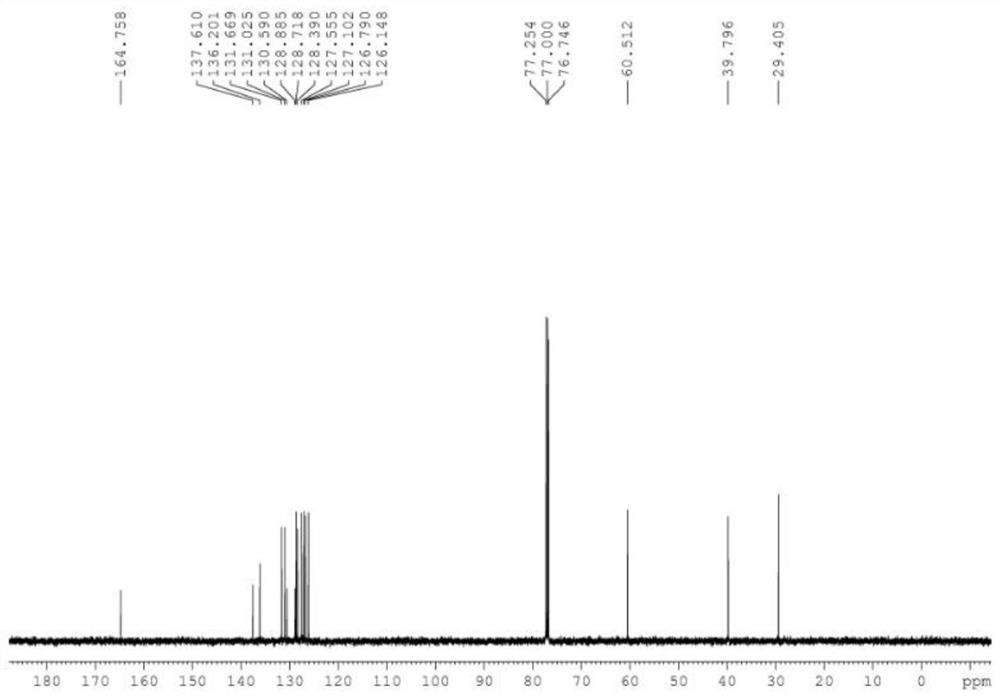
[0038] 4a 1 H NMR spectrum see figure 1 , 13 C NMR spectrum see figure 2 .
[0039] 5,13a-Dihydro-6H,8H-benzo[5,6][1,3]selenazino[2,3-a]isoquinolin-8-one 13-oxide(4a)White solid(81%);mp:83 -85°C;
[0040] 1 H NMR (CDCl 3 ,500MHz)δ8.19-8.17(d,J=8.0Hz,1H),7.42-7.38(m,2H),7.34-7.29(m,4...
Embodiment 2
[0043] Embodiment 2: the preparation of product 4b
[0044]At room temperature, add 10mmol of 1,5-methyl-2,3,4-tetrahydroisoquinoline, 15mmol of sodium selenide and 10mmol of 2-bromobenzaldehyde in a 50mL round bottom flask, and then add 30mL of DMSO, 1 mmol of magnesium acetate and 1 mmol of copper acetate, and 20 mmol of cesium carbonate were stirred at 100°C for 24 hours. After cooling, add 20 mL of saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 20 mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain The pure product of benzo-6,8-dihydroisoquinoline-1-selensulfonylbenzamide compound 4b.
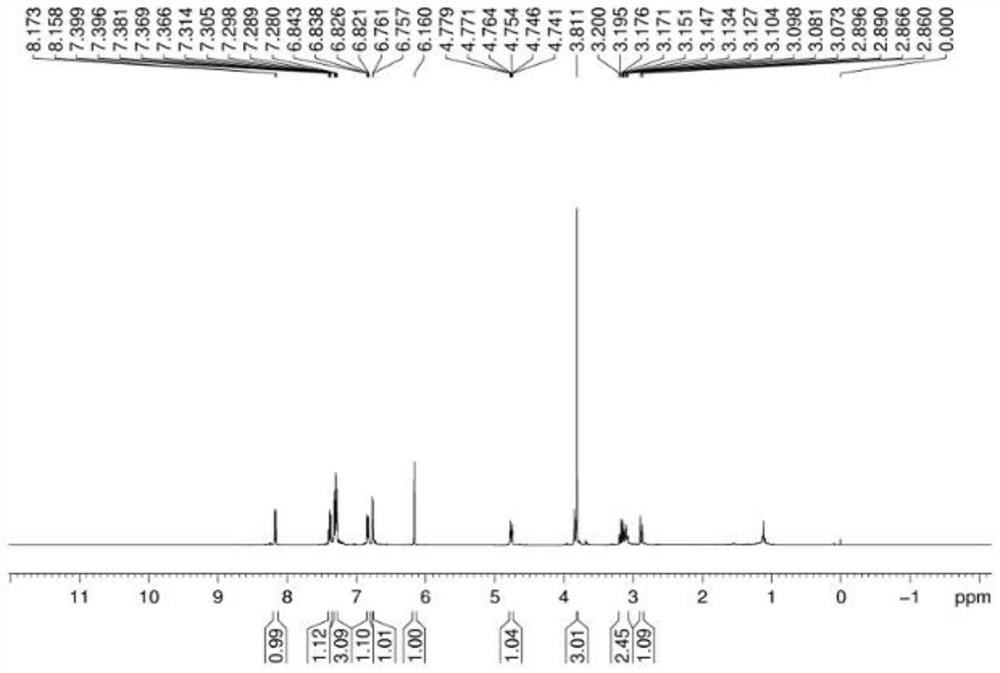
[0045] 4b 1 H NMR spectrum see image 3 , 13 C NMR spectrum see Figure 4 .
[0046] 3-Methoxy-5,13a-dihydro-6H,8H-benzo[5,6][1,3]selenazino[2,3-a]isoquinolin-8-one 13-oxide(4b)Yellow oil(73%) ;
[0047] 1 H NMR (CDCl 3 ,500MHz)δ8.16(d,J=7.5Hz,1H),7.38(dd,J=7.5Hz,1.5Hz,1H),7.3...
Embodiment 3
[0049] Embodiment 3: the preparation of product 4c
[0050] At room temperature, in a 50mL round bottom flask, add 10mmol 1,2,3,4-tetrahydroisoquinoline, 15mmol sodium selenide and 10mmol 2-bromo-2-methylbenzaldehyde, and then add 30mL DMSO, 1 mmol of magnesium acetate and 1 mmol of copper acetate, and 20 mmol of cesium carbonate were stirred at 100°C for 24 hours. After cooling, add 20mL saturated aqueous sodium chloride solution to the system, extract 3 times with ethyl acetate, 20mL each time, combine the organic phases, dry with anhydrous sodium sulfate, evaporate the solvent, and obtain by 200-300 mesh silica gel column chromatography The pure product of benzo-6,8-dihydroisoquinoline-1-selensulfonylbenzamide compound 4c.
[0051] 4c 1 H NMR spectrum see Figure 5 , 13 C NMR spectrum see Image 6 .
[0052] 12-Methyl-5,13a-dihydro-6H,8H-benzo[5,6][1,3]selenazino[2,3-a]isoquinolin-8-one 13-oxide(4c)Pale yellow solid(89% );mp:75-77℃;
[0053] 1 H NMR (CDCl 3 ,500MH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com