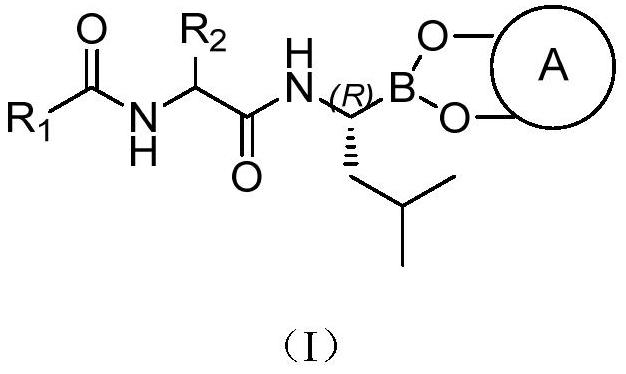
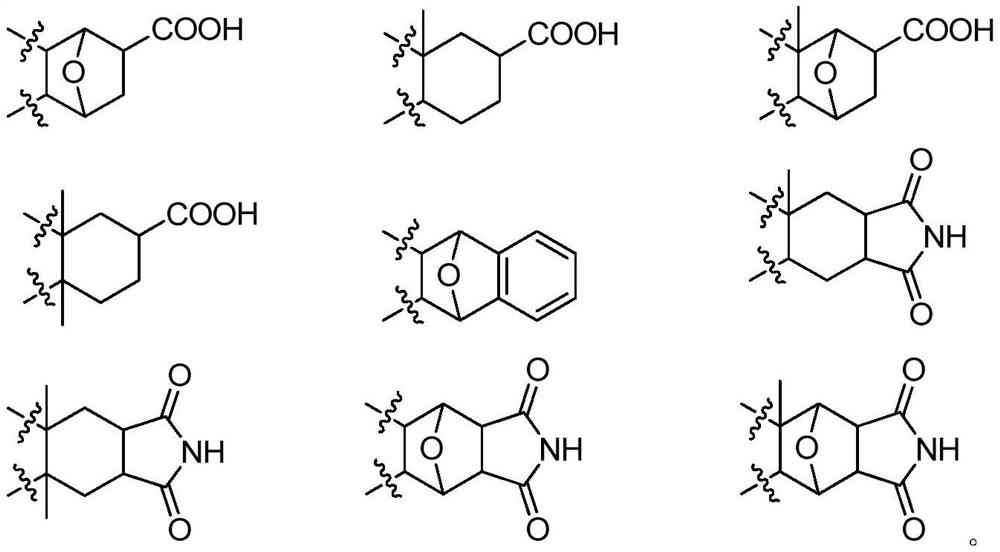
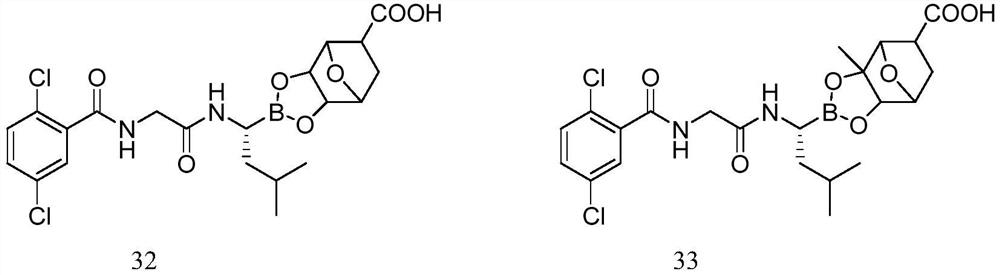
Borate compound and pharmaceutically acceptable salt thereof, and preparation method and application of boric acid ester compound and pharmaceutically acceptable salt thereof
A technology of boric acid esters and compounds, applied in the field of drug synthesis, can solve problems such as toxic and side effects, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: The preparation of the compound of the present invention can be implemented according to the following process:
[0054] Preparation of compound (II)
[0055]
[0056] 1. Preparation of compound (II-3):
[0057] Dissolve compound (II-1), HOBt in anhydrous DCM (dichloromethane) and stir at -5°C for 10 min, then add EDCI·HCl at this temperature, stir for 15-20 min, then add compound (II-2) Stir for 15-20 minutes, then add DIPEA and stir for 20 minutes, then move to room temperature for reaction. After the reaction is complete, pour it into water, and use dilute HCl, NaHCO 3 The solution was washed, the combined organic phases were washed with saturated brine, extracted with DCM, dried over anhydrous sodium sulfate, and evaporated to dryness to obtain compound (II-3).
[0058] 2. Preparation of compound (II-4):
[0059] Compound (II-3) was dissolved in anhydrous DCM, TFA was slowly added dropwise at -5°C, stirred for 0.5 hours, then raised to room temp...
Embodiment 2
[0118] Example 2: Determination of inhibition of proteasome activity
[0119] 1. Proteasome inhibitory activity
[0120] The present invention utilizes fluorescent polypeptide substrate Suc-Leu-Leu-Val-Tyr-AMC (abbreviation Suc-LLVY-AMC, Suc represents succinyl, AMC represents 7-amide-4-methylcoumarin) to measure proteasome Chymotrypsin-like enzyme activity.
[0121] The proteasome used in the present invention is human erythrocyte 20S proteasome, and the enzyme, fluorescent substrate and test buffer are all purchased from Enzo Company. The experimental system was 16 μL, including 8 μL of substrate, 4 μL (0.8 ng) of proteasome, the final concentration was 50 μM, and 4 μL of drug (inhibitor), the final concentration was 2×10 -6 M~4.88×10 -10 M, the last concentration is 0M, the actual configuration concentration is 8×10 -6 M~1.95×10 -9 M, the last concentration is 0M. The specific experimental process is as follows:
Embodiment 3
[0135] Embodiment 3: compound stability test
[0136] 1. Stability of compounds in simulated gastric juice, intestinal juice and pH6.8 phosphate buffer
[0137] In the experiment, LC-MS was used to detect the peptide boronic acid fragments obtained by decomposition at different time points in different pH solutions, and the content of the fragments corresponded to the decomposition amount of the parent compound.
[0138] 1. Each pH buffer solution configuration: pH1.2: add 8-9ml (8.33ml) concentrated hydrochloric acid to dilute to 1000ml with water; pH4.5: take 7.7g of ammonium acetate, add 50ml of water to dissolve, add 6ml of glacial acetic acid, and then dilute to volume to 100ml; pH6.8: Take 250ml of 0.2mol / L potassium dihydrogen phosphate solution, add 118ml of 0.2mol / L sodium hydroxide solution, dilute to 1000ml with water, and shake well.
[0139] 2. Compound preparation: The compound was prepared with different pH buffer solutions to a concentration of 1 mg / ml, and pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com