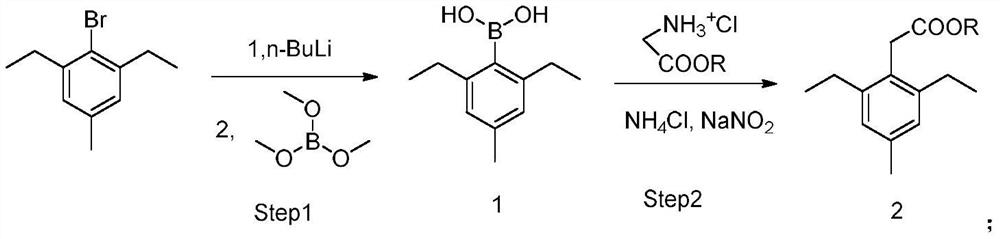
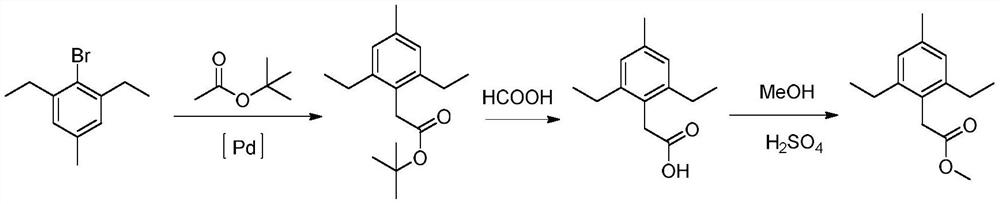
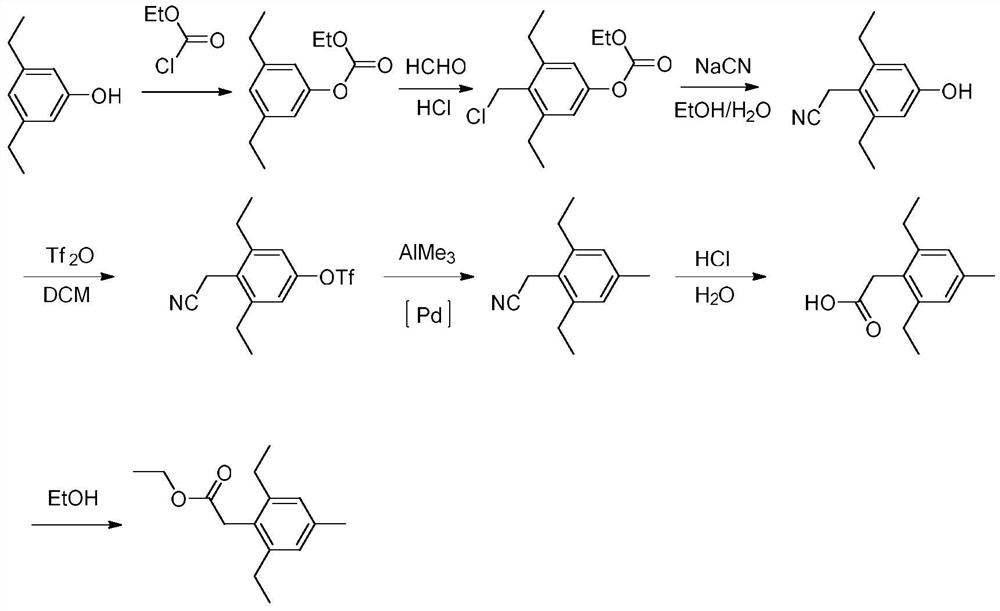
Method for synthesizing 2,6-diethyl-4-methylphenylacetate
A technology of methyl phenylacetate and methyl phenylboronic acid, which is applied in the field of organic synthesis, can solve the problems of expensive catalysts, complex reaction types, and harsh equipment requirements, and is suitable for large-scale industrial production, avoiding precious metal catalysts, and simplifying the production process. The effect of craft
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 500mL dry four-necked flask, vacuumize and replace the system with nitrogen three times, then add 200mL of anhydrous tetrahydrofuran and 40g of 2,6-diethyl-4-methylbromobenzene (molecular weight 227.15, 176.1mmol , 1eq), the temperature was cooled to -65°C in a dry-ice acetone bath, and 84.5mL of n-butyl lithium in n-hexane (2.5mol / L, 211.31mmol, 1.2eq) was slowly added dropwise. After about 40 minutes of dripping, continue stirring at -65°C for 0.5h. Then slowly add 27.45g of borane trimethyl ester (molecular weight 103.91, 264.14mmol, 1.5eq) dropwise, and drop it for about 30 minutes, then continue to stir at -65°C for 0.5h, then let the dry ice acetone bath volatilize naturally to raise the temperature of the reaction solution Stir overnight at room temperature. Add 100 mL of 1 mol / L dilute hydrochloric acid to quench the reaction solution, then extract twice with 100 mL of ethyl acetate, combine the organic phases and concentrate to dryness to obtain a crude p...
Embodiment 2
[0038] In a 500mL dry four-necked flask, vacuumize and replace the system with nitrogen three times, then add 200mL of anhydrous tetrahydrofuran and 40g of 2,6-diethyl-4-methylbromobenzene (molecular weight 227.15, 176.1mmol , 1eq), the temperature was cooled to -65°C in a dry-ice acetone bath, and 74.0 mL of n-butyl lithium in n-hexane (2.5 mol / L, 184.9 mmol, 1.05 eq) was slowly added dropwise. After about 35 minutes of dripping, continue to stir at -65°C for 0.5h. Then slowly add 20.13g borane trimethyl ester (molecular weight 103.91, 193.7mmol, 1.1eq) dropwise, and the dropwise is completed in about 20 minutes. After the dropwise operation, continue to stir at -65°C for 0.5h, then let the dry ice acetone bath volatilize naturally to raise the temperature of the reaction solution. Stir overnight at room temperature. Add 100 mL of 1 mol / L dilute hydrochloric acid to quench the reaction solution, then extract twice with 100 mL of ethyl acetate, combine the organic phases and ...
Embodiment 3
[0042] In a 1000mL dry four-neck flask, vacuumize and replace the system with nitrogen three times, then add 200mL of anhydrous tetrahydrofuran and 40g of 2,6-diethyl-4-methylbromobenzene (molecular weight 227.15, 176.1mmol , 1eq), the temperature was cooled to -65°C in a dry-ice acetone bath, and 105.6mL of n-butyl lithium in n-hexane (2.5mol / L, 264.1mmol, 1.5eq) was slowly added dropwise. After about 50 minutes of dripping, continue stirring at -65°C for 0.5h. Then slowly add 36.60g of borane trimethyl ester (molecular weight 103.91, 352.2mmol, 2.0eq) dropwise, and drop it in about 45 minutes, continue stirring at -65°C for 0.5h, then let the dry ice acetone bath volatilize naturally to raise the temperature of the reaction solution Stir overnight at room temperature. Add 150 mL of 1mol / L dilute hydrochloric acid to quench the reaction solution, then extract twice with 100 mL of ethyl acetate, combine the organic phases and concentrate to dryness to obtain a crude product, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com